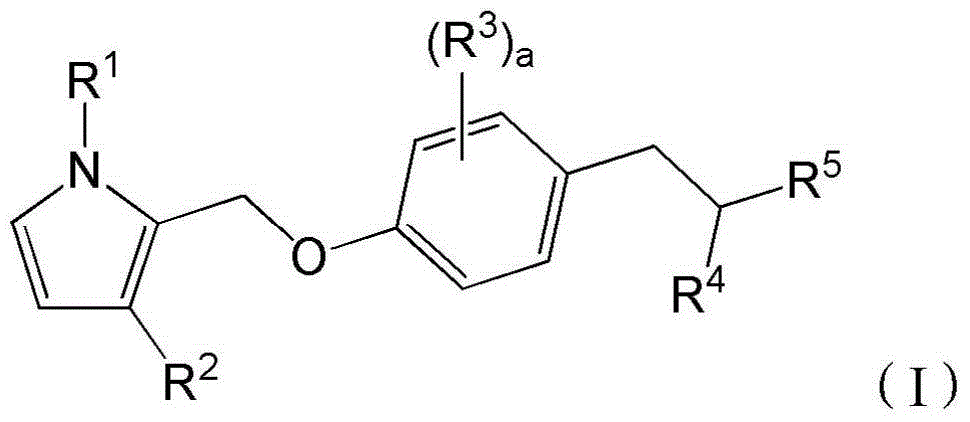
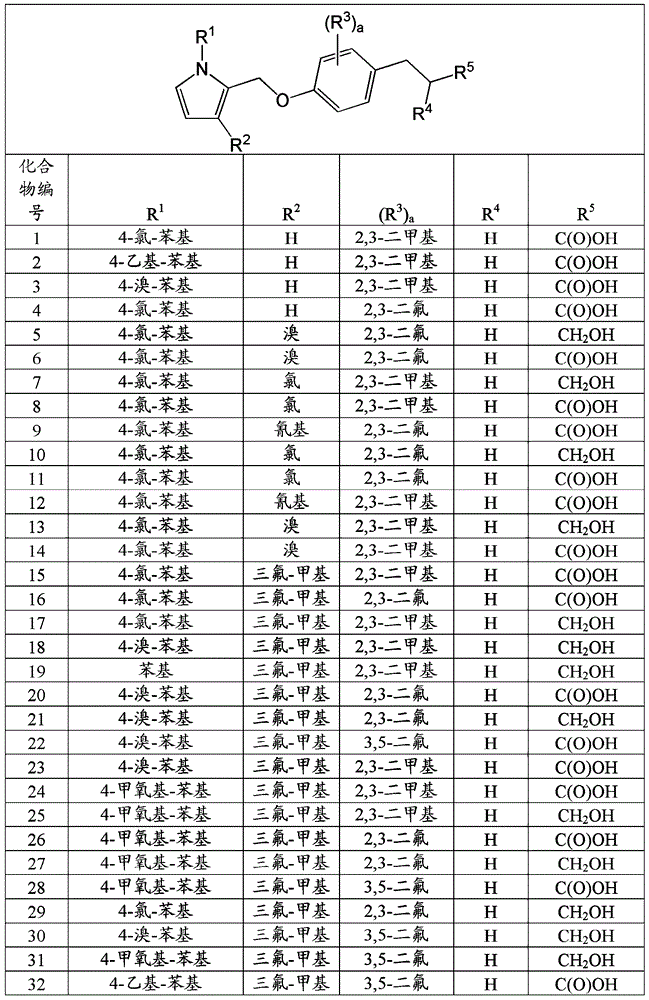
Bicyclic pyrrole derivatives useful as agonists of gpr120
A compound, pyridyl-based technology, for the treatment of GPR120-mediated disorders and conditions, which can address the potential to increase CLP-1, among other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0180] The following examples are given to aid in the understanding of the invention and are not intended and should not be construed as limiting in any way the invention as set forth in the claims that follow the examples.
[0181] In the following examples, some synthesis products that have been isolated as residues are listed. Those skilled in the art will appreciate that the term "residue" does not limit the physical state of the product when it is isolated, and may include, for example, solids, oils, foams, gums, slurries, and the like.
example 1
[0183] 3-(4-{[1-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrrol-2-yl]methoxy}-3,5-difluorophenyl) Propionic acid (Compound #70)
[0184]
[0185] Step A: 1-(Benzenesulfonyl)-3-(trifluoromethyl)-1H-pyrrole-2-carboxylic acid methyl ester
[0186]
[0187] in N 2 3-Bromo-1-(benzenesulfonyl)-1H-pyrrole-2-carboxylic acid methyl ester (3.07g, 8.0mmol, 1 equivalent), 2,2-difluoro-2-(fluorosulfonyl)acetic acid A suspension of the methyl ester (6.32 mL, 48.1 mmol, 6 equiv) and CuI (1.53 g, 8.0 mmol, 1 equiv) in NMP (20 mL) was heated to 80 °C overnight. Additional methyl 2,2-difluoro-2-(fluorosulfonyl)acetate (6.32 mL, 48.1 mmol, 6 equiv) and CuI (1.53 g, 8.0 mmol, 1 equiv) were added and the resulting mixture was heated overnight. The resulting brown suspension was filtered through CELITE with diethyl ether, water, saturated NaHCO 3 , brine washing the diethyl ether solution, and then through MgSO 4 Dried and then concentrated. The resulting residue was purified by fla...
example 2
[0211] 3-(4-{[1-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrrol-2-yl]methoxy}-2,3-dimethylbenzene base) propan-1-ol (compound #46)
[0212]
[0213] at 0°C in N 2 Next, to a solution of the product prepared in Example 1, Step E (46 mg, 0.10 mmol, 1 eq) in THF (2 mL) was added LAH (0.20 mL of 1M in THF, 0.20 mmol, 2 eq). After 1 hour, firstly saturated potassium sodium tartrate (0.5 mL) was added dropwise, the solution was warmed to room temperature, diethyl ether was added, washed with brine, washed over MgSO 4 Dried and concentrated. The resulting residue was purified by flash chromatography (4 g column) eluting with 10 to 30% EtOAc / heptane to give 3-(4-{[1-(4-chlorophenyl)-3-(trifluoro Methyl)-1Hpyrrol-2-yl]methoxy}-2,3-dimethylphenyl)propan-1-ol.
[0214] 1 H NMR (300MHz, CD 3 OD)δ: 7.56(s, 4H), 7.05(d, J=3.0Hz, 1H), 6.76-6.83(m, 2H), 4.49(d, J=3.0Hz, 1H), 5.00(s, 2H) , 3.55 (t, J=5.7Hz, 2H), 2.61-2.66 (m, 2H), 1.71-1.84 (m, 2H). Mass Spectrum (ESI, m / z): For C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com