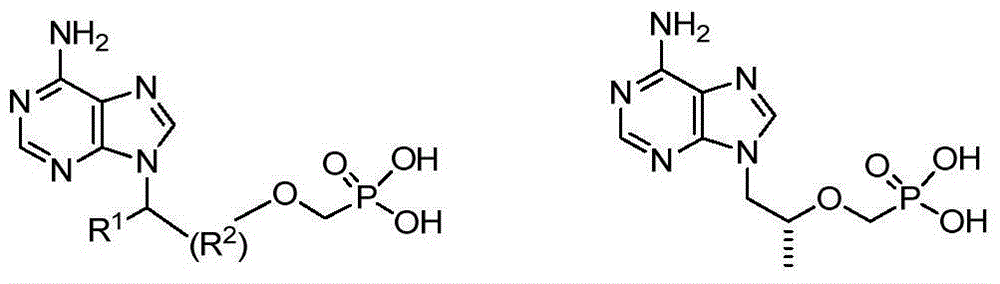
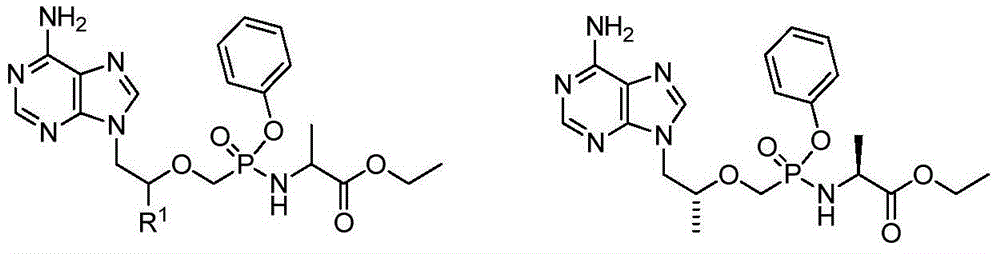
Aryl-substituted phosphonaminate and application in medical science thereof
A substituent and pharmaceutical technology, applied in the field of phosphoramide derivatives, can solve the problems of poor biofilm penetration ability and poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: (2S)-2-[[[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-phenoxy-phosphoryl]amino ]propionic acid-2-(2-methoxyethoxy)ethyl ester (compound 1)
[0117] 2-(2-methoxyethoxy)ethyl(2S)-2-[[[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-phenoxy-phosphoryl]amino]propanoate
[0118]
[0119] The first step: (2S)-2-(tert-butoxyamino)propionic acid-2-(2-methoxyethoxy)ethyl ester (1B)
[0120] 2-(2-methoxyethoxy)ethyl(2S)-2-(tert-butoxycarbonylamino)propanoate
[0121]
[0122] (2S)-2-(tert-butoxyamino)propionic acid (6.0g, 0.05mol), 2-(2-methoxyethoxy)ethanol (9.5g, 0.05mol) and toluene dichloro (150 mL), then dicyclohexylcarbodiimide (10.3 g, 0.05 mol) and 4-dimethylaminopyridine (6.1 g, 0.05 mol) were added, and the reaction was stirred overnight at room temperature under a nitrogen atmosphere. The temperature was raised to react overnight. The reaction solution was filtered, the filter cake was washed with saturated brine, dried over anhydrous sodiu...
Embodiment 2
[0135] Embodiment 2: the resolution of compound 1
[0136] Take (2S)-2-[[[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-phenoxy-phosphoryl]amino]propanoic acid - 2-(2-Methoxyethoxy)ethyl ester (compound 1) (200 mg) was used for resolution.
[0137] Preparation conditions: Instrument: Gilson GX-281; Column: CHIRALPAK AD-H, 20×250mm, 5μm; Mobile phase: A: n-hexane, B: isopropanol; Isocratic elution: A:B=50:50 (v / v); Flow rate: 6mL / min; Wavelength: 260nm; ml; injection: 20 μL / needle.
[0138] After separation, the fractions were concentrated and dried via a rotary evaporator at 40°C to obtain two optical isomers Compound 1-1 (50 mg) and Compound 1-2 (45 mg).
[0139] Compound 1-1
[0140] 1 H NMR (400MHz, CDCl 3 )δ8.32(s,1H),7.97(s,1H),7.34-7.28(m,2H),7.17-7.14(m,3H),5.80(s,2H),4.43(dd,1H),4.26 -4.14(m,3H),4.13-4.05(m,1H),4.05-4.00(m,1H),4.00-3.94(m,1H),3.69–3.61(m,5H),3.57-3.52(m, 2H), 3.44(t, 1H), 3.37(s, 3H), 1.25(d, 3H), 1.21(s, 3H).
[0141] MS M / Z (ESI): 537....
Embodiment 3
[0147] Example 3: (2S)-2-[[[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-phenoxy-phosphoryl]amino ]propionate-2-(2-hydroxyethoxy)ethyl ester (compound 2)
[0148] 2-(2-hydroxyethoxy)ethyl(2S)-2-[[[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-phenoxy-phosphoryl]amino]propanoate
[0149]
[0150] The first step: 2-(2-benzyloxyethoxy)ethanol (2B)
[0151] 2-(2-benzyloxyethoxy)ethanol
[0152]
[0153] 2-(2-Methoxyethoxy)ethanol (2A) (21.2 g, 0.2 mol) and cesium carbonate (8.55 g, 0.05 mol) were dissolved in N,N-dimethylformamide (50 mL), Add benzyl bromide (27.7 g, 0.085 mol). Stir at room temperature for 3 days. Water (300mL) was added to the reaction solution, extracted with ethyl acetate (100mL×2), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum Ether / ethyl acetate (v / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com