EGFR inhibitor and preparing method and application thereof
An epidermal growth factor and inhibitor technology, applied in the field of medicine, can solve problems such as drug failure and drug resistance in patients, and achieve the effect of solving drug resistance problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
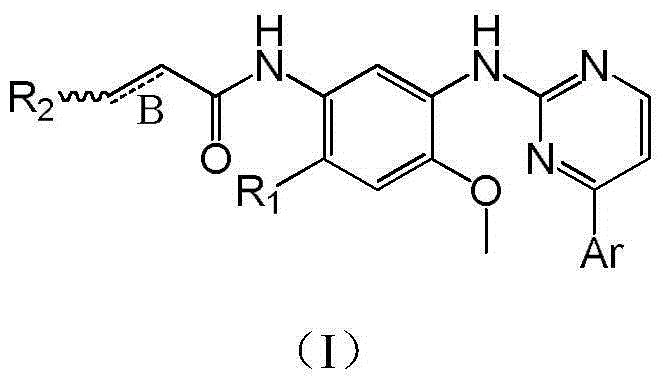
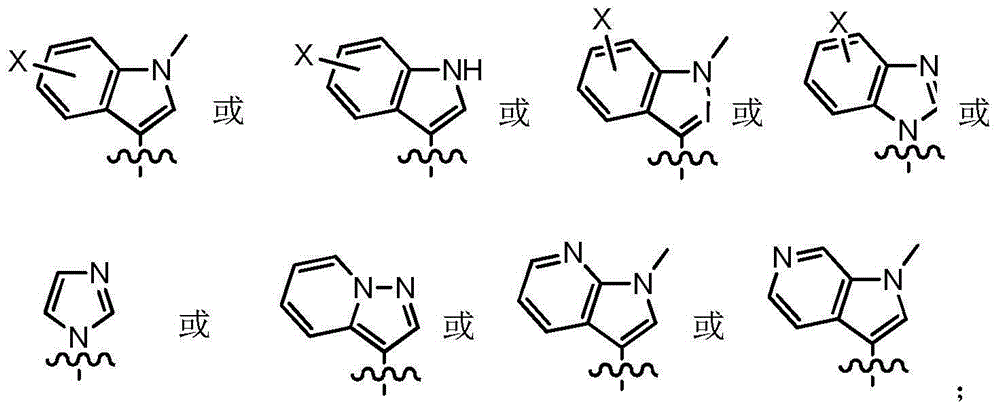
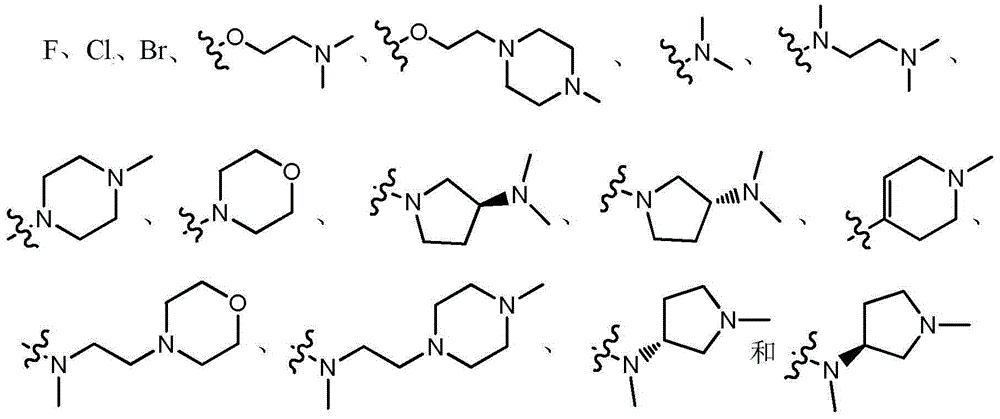
[0033] Example 1: 2-{4-fluoro-2-methoxy-5-[N,N-dimethylaminomethyl-(E)-acrylamido]}anilino-4-(1-methyl- Preparation of 3-indolyl)pyrimidine (compound 101)
[0034]
[0035] Preparation of 3-(2-chloro-4-pyrimidinyl)-1-methylindole
[0036]
[0037] 2,4-Dichloropyrimidine (450g, 3.02mol) was dissolved in ethylene glycol dimethyl ether (6L), stirred in an ice bath, aluminum chloride was added in batches, and then stirred and reacted at room temperature for half an hour. N-methylindole (400 g, 3.05 mol) was added dropwise. Then heated to 80°C and stirred for 4 hours. Liquid chromatography-mass spectrometry (LCMS) detected that the reaction was complete. Stop the reaction, cool down to room temperature, slowly introduce the reaction solution into ice water (18 L), stir rapidly, a large amount of orange-red solid is precipitated, filter with suction, wash the filter cake with cold water three times, and dry the obtained filter cake to obtain a light red solid crude product ...
Embodiment 2
[0053] Example 2: 2-{4-N,N-dimethylamino-2-methoxy-5-[N,N-dimethylaminomethyl-(E)-acrylamido]}anilino- Preparation of 4-(1-methyl-3-indolyl)pyrimidine (compound 102)
[0054]
[0055] Preparation of 2-(4-N,N-Dimethylamino-2-methoxy-5-nitro)anilino-4-(1-methyl-3-indolyl)pyrimidine
[0056]
[0057] 2-(4-Fluoro-2-methoxy-5-nitro)anilino-4-(1-methyl-3-indolyl)pyrimidine (400 mg, 1.012 mmol) was dissolved in N,N-di Dimethylamine (50mg, 1.113mmol) and N,N-diisopropylethylamine (500mg, 4.048mmol) were added to methylformamide (5ml), and the reaction was stirred at 86°C for 3 hours. LCMS detected that the reaction was complete. Cool to room temperature, add the reaction solution dropwise to stirring water, a large amount of solids are precipitated, filter with suction, wash the filter cake with a small amount of water, dissolve the filter cake in dichloromethane, add dropwise to stirring petroleum ether for beating treatment, the obtained solid is pumped Filter dry. Obtaine...
Embodiment 3
[0064] Example 3: 2-{4-(1-methyl-N,N-dimethylaminoethyl)amino-2-methoxy-5-(3'-N,N-dimethylamine Preparation of methyl-acryloyl)-amino}anilino-4-(1-methyl-3-indolyl)pyrimidine (compound 103)
[0065]
[0066] 2-{4-(1-methyl-N,N-dimethylaminoethyl)amino-2-methoxy-5-nitro}anilino-4-(1-methyl-3- Preparation of indolyl)pyrimidine
[0067]
[0068] 2-(4-Fluoro-2-methoxy-5-nitro)anilino-4-(1-methyl-3-indolyl)pyrimidine (Intermediate A) (150 g, 0.381 mol) was dissolved in Dimethylsulfoxide (3L), stirred at room temperature, added N,N,N-trimethylethylenediamine (55g, 0.534mol) and N,N,-diisopropylethylamine (123g, 0.953 mol), then heated to 86°C, and stirred for three hours. LCMS detected that the reaction was complete. Stop the reaction, cool to room temperature, slowly add the reaction solution into stirring ice water (10L), a large amount of orange-red solid precipitates, filter with suction, and dry the filter cake to obtain 170g of solid, purity: 98%, yield: 94% . LC-M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com