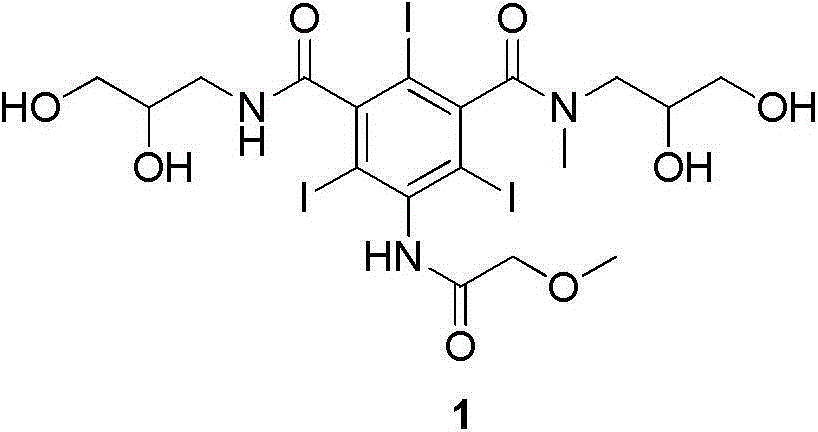
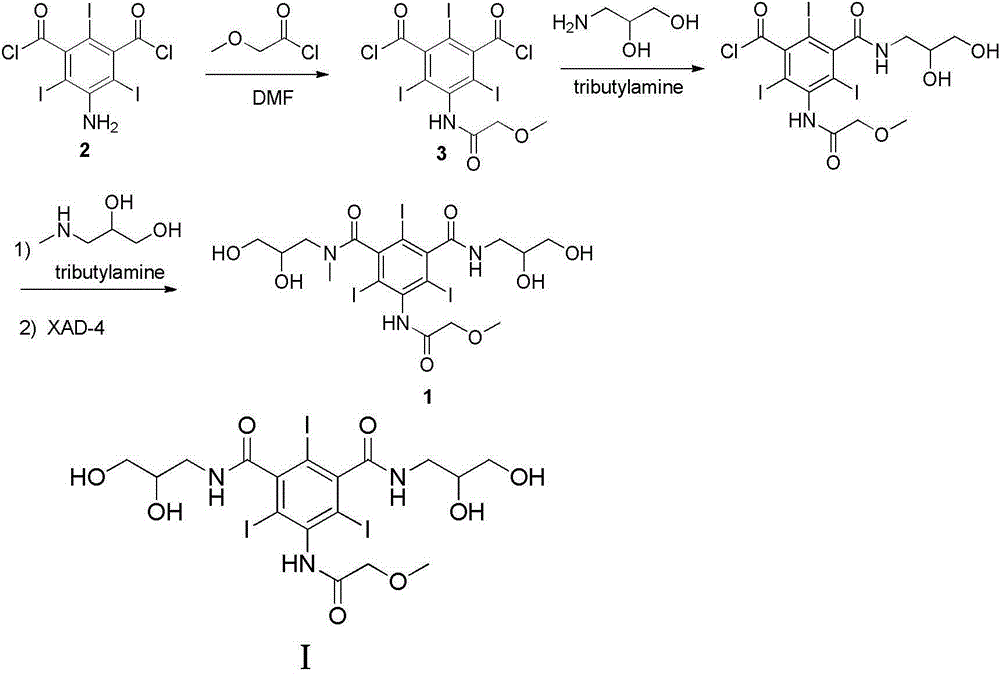
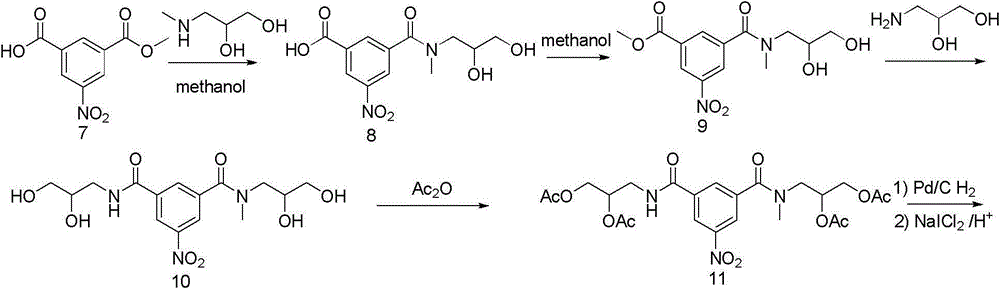
Iopromide preparation method
A technology of iopromide and amidation, which is applied in the field of medicine and can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of 5-methoxyacetyl-2,4,6-triiodoisophthaloyl chloride (3)
[0036] Dissolve 38g (0.35mol) of methoxyacetyl chloride in 20mL of N,N-dimethylacetamide, and drop into 60g (0.1mol) of N,N-dimethylacetamide in an ice bath Acetamide solution (100 mL), after dropping, stirred at room temperature for 20 h. After the reaction was completed, the reaction solution was poured into ice water, and a white solid was precipitated, which was filtered by suction. The solid was dissolved in dichloromethane, washed with water (200mL×3), saturated sodium bicarbonate solution (400mL×2), saturated sodium chloride solution (200mL×2), dried over anhydrous sodium sulfate, and evaporated to dryness , a white solid was obtained. Yield 86%. HPLC purity 98%. mp: 199-201°C.
[0037] MS: 667.2 [M-H] -
[0038] 1 H-NMR (300MHz, DMSO) δ4.02(s, 2H), 3.49(s, 3H).
Embodiment 2
[0039] Example 2: Preparation of 3-methoxyacetyl-5-(2,3-dihydroxy-N-methyl n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (4)
[0040] Dissolve 8.3g (0.079mol) of 3-methylamino-1,2-propanediol and 15.6g (0.084mol) of tri-n-butylamine in 40mL of N,N-dimethylacetamide, drop Put 75g (0.11mol) of intermediate (3) in N,N-dimethylacetamide (150mL) solution, dropwise, react at room temperature for 3h. After the reaction, evaporate the solvent to dryness, add water at 0°C, stir in an ice bath for 0.5 h, filter with suction, wash the filter cake with a small amount of cold water, adjust the pH of the aqueous solution to 9, and separate and purify the resulting aqueous solution with an anion exchange resin to obtain a light white solid. Yield 46%. HPLC purity 99%. mp: 163-165°C.
[0041] MS: 719.4[M+H] + , 740.5[M+Na] + , 716.5[M-H] - , 754[M+Cl] -
[0042] 1 H-NMR (300MHz, DMSO) δ9.79(s, 1H), 4.76(s, 1H), 4.59(s, 1H), 3.96(s, 2H), 3.88(s, 1H), 3.66(d, J =15.6Hz, 1H), 3.45(s, 5H...
Embodiment 3
[0043] Example 3: Preparation of 3-methoxyacetyl-5-(2,3-diacetoxy-N-methyl n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (5)
[0044] Add 25g (0.035mol) of intermediate (4) to 125mL of acetic anhydride and 50mL of glacial acetic acid, add 0.3g (0.0017mol) of p-toluenesulfonic acid at 0-5°C, and stir at room temperature for 10h. After the reaction, the solvent was evaporated to dryness, and an appropriate amount of absolute ethanol was added to the residue, and rotary evaporation was continued to obtain a light white solid. Yield 80%. mp: 108-110°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com