Method for synthesizing nitrogen-doped graphene hydrogel in one step and using nitrogen-doped graphene hydrogel for electrically adsorbing heavy metal ions in water
A technology of nitrogen-doped graphene and heavy metal ions is applied in the directions of alkali metal compounds, water/sewage treatment, water/sludge/sewage treatment, etc., to achieve the effects of simple and easy preparation method, environmental protection and pollution-free preparation process, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of nitrogen-doped hydrogel material paper electrode includes the following steps:
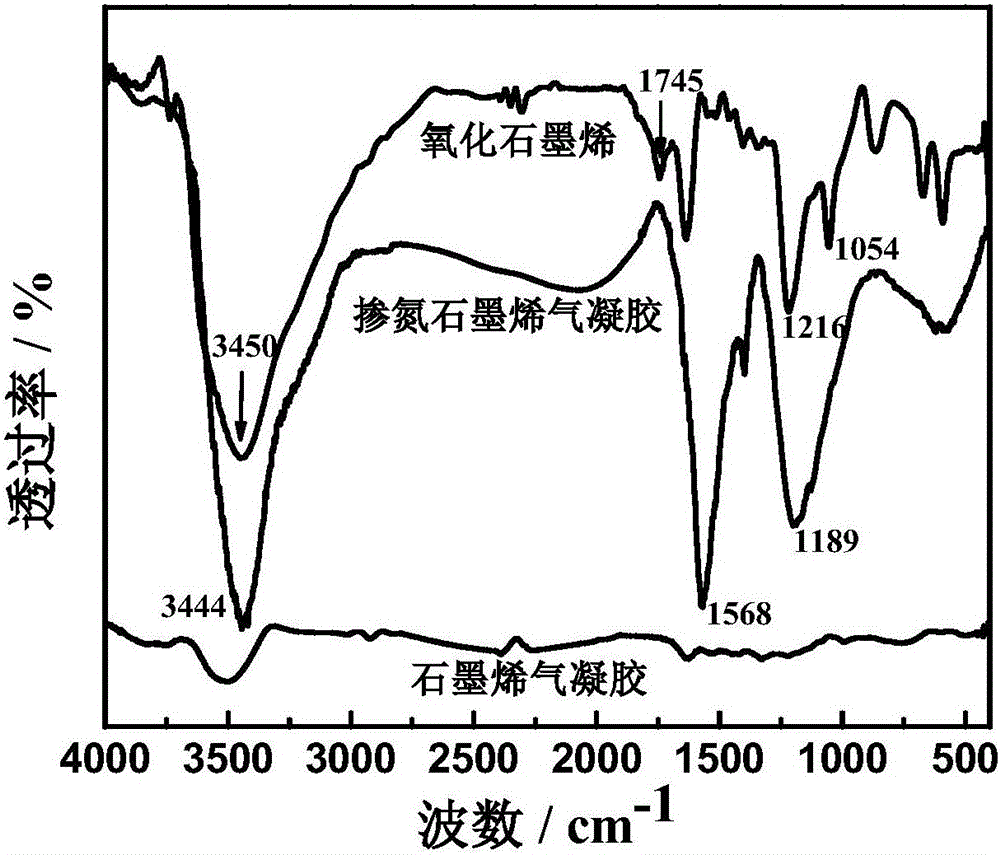
[0024] (1) 0.2 g of graphene oxide (GO) was ultrasonically dispersed in 100 ml of distilled water, and then 6 g of urea was added, and mechanically stirred for 15 min. The mixture was poured into a hydrothermal reactor and heated to 180°C for 12 hours. The resulting product was immersed in distilled water for 3 to 4 days, and finally the sample was freeze-dried at -50°C for 24 hours to obtain nitrogen-doped graphene airgel.
[0025] (2) Add 90 mg of the nitrogen-doped graphene airgel material prepared in step (1) into 2 mL of 4 wt % polyvinyl alcohol solution, and ultrasonically disperse the composite material in the solution evenly. Take 0.16mL of the above dispersion liquid and apply it evenly on a 20mm×5mm hard paper sheet (thickness 400μm), and freeze-dry it at -50°C for 4h to make a nitrogen-doped graphene airgel paper electrode.
[0026] The prepared nitrogen-dope...
Embodiment 2
[0028] The preparation process of the nitrogen-doped graphene airgel paper electrode is the same as that of Example 1.
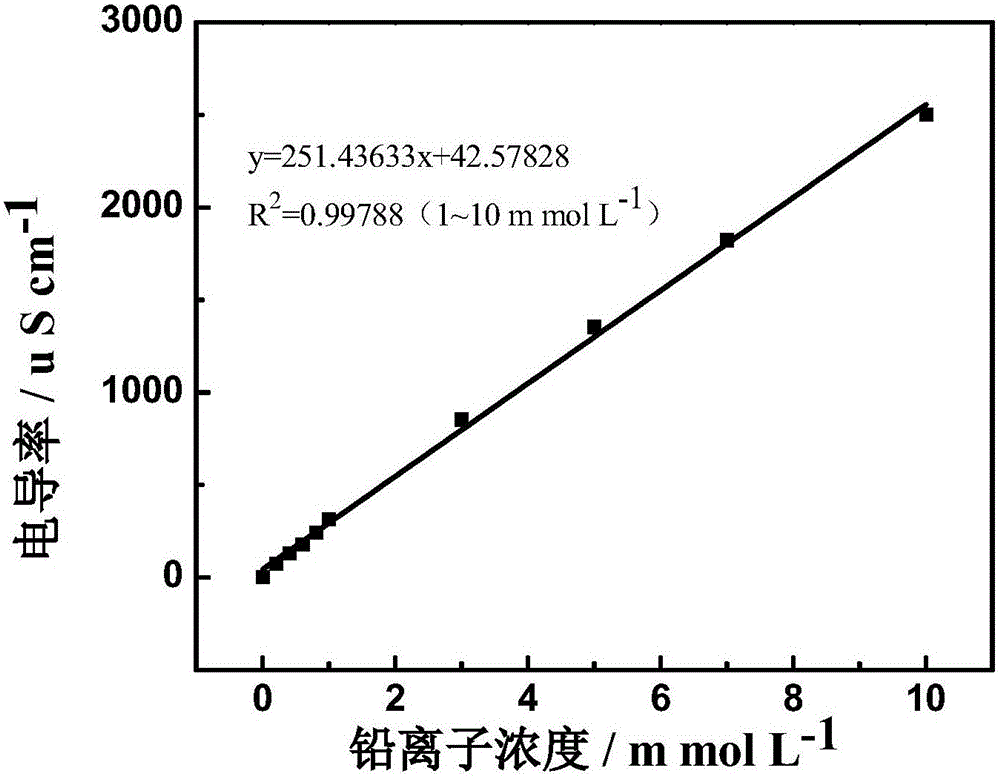
[0029] Cyclic electrosorption experiments were performed on nitrogen-doped graphene airgel paper electrodes. The nitrogen-doped graphene airgel paper electrode was placed in 80 mL of Pb(NO 3 ) 2 In the solution, apply a potential of -0.3V, and record the conductivity of the solution, record the conductivity of the solution again after 2 minutes, and calculate the removal rate. Then the potential was removed to allow it to desorb, and the cycle was repeated several times. Experimental results such as Figure 4 shown. The first adsorption of Pb 2+ The removal rate is 75%, and after 100 cycles of use, the electrode is on Pb 2+ The removal rate was 70%. It shows that the material has extremely high regeneration performance.
Embodiment 3
[0031] The preparation process of the nitrogen-doped graphene airgel paper electrode is the same as that of Example 1.
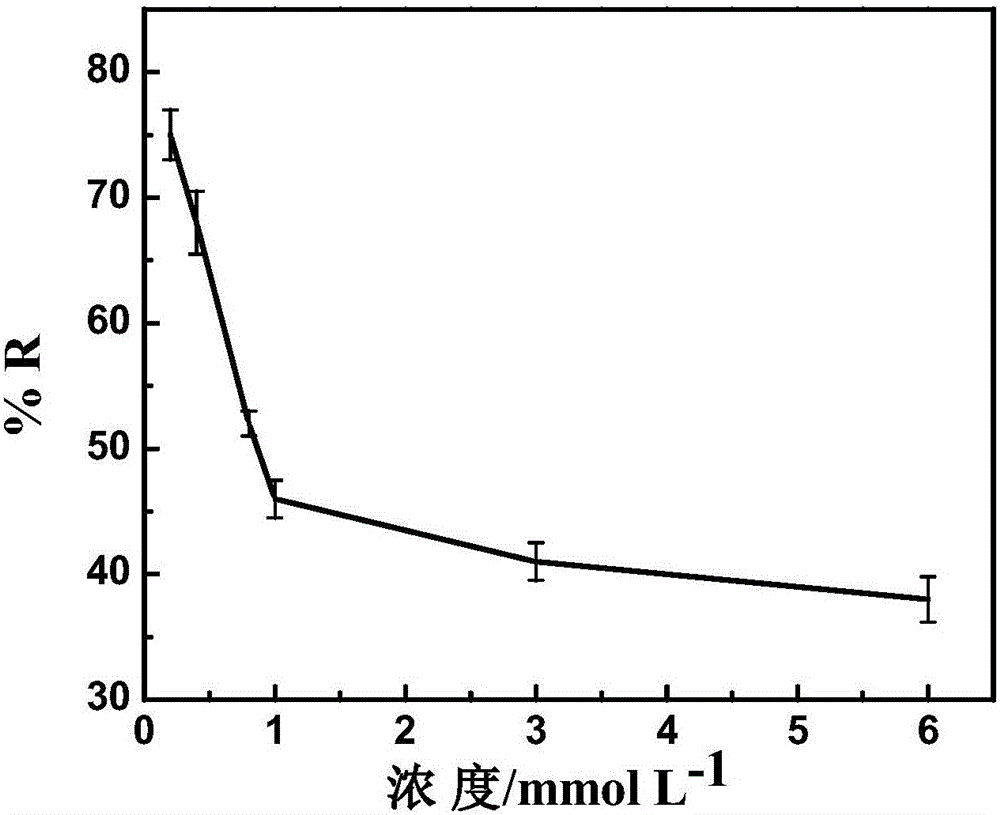
[0032] The prepared nitrogen-doped graphene airgel paper electrode was used in 3mM Pb(NO 3 ) 2 For the electrochemical treatment of the solution, the applied voltage was -0.3V, and the treatment time was 2min. Such as Figure 5 As shown, the nitrogen-doped graphene airgel paper electrode paired with Pb 2+ The removal rate is 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com