Hexabenzocoronene compound containing thioether in swallowtail side chain and preparation method thereof
A dovetail-shaped, sulfide-containing technology, used in sulfide preparation, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problem of high temperature range of liquid crystal phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
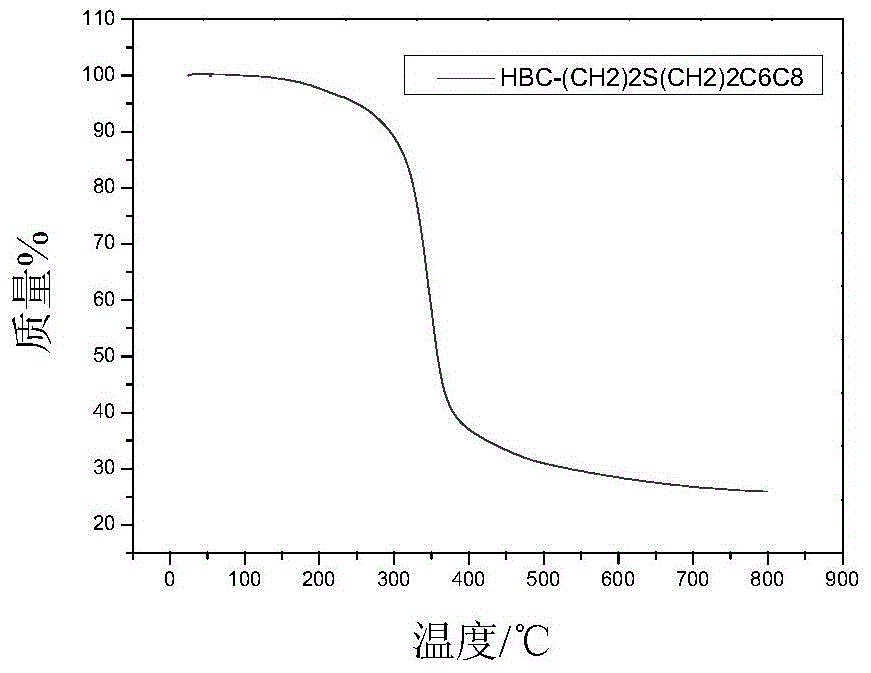
[0075] Embodiment 1: prepare HBC-(CH 2 ) 2 SCH 2 CH(C 6 h 13 )C 8 h 17
[0076] (1) p-bromophenethylmercaptan
[0077] Put p-bromophenethyl bromide (86.87g, 0.33mol), thiourea (30.14g, 0.396mol), and absolute ethanol (120ml) in a round bottom flask, mix and heat to reflux until a white precipitate precipitates, and after filtration , placed in a single-necked bottle and added NaOH (135ml, 5M) aqueous solution, heated under reflux for five hours, after acidification with dilute hydrochloric acid, separated the mercaptan layer, dried over anhydrous sodium sulfate, and distilled under reduced pressure on a 20cm Weiss fractionation column (distillation range 146- 148°C). P-bromophenethyl bromide can be obtained by passing 2-(4'-bromophenyl)ethanol into hydrogen bromide.
[0078] Through NMR analysis, what is obtained is p-bromophenethylmercaptan, and the specific data are: 1 HNMR (300MHz, CDCl 3 ): δ(ppm) δ1.37(t,1H,-),2.76(t,2H,-CH 2 -),2.86(t,2H,-CH 2 -),7.07(d,2H,A...
Embodiment 2
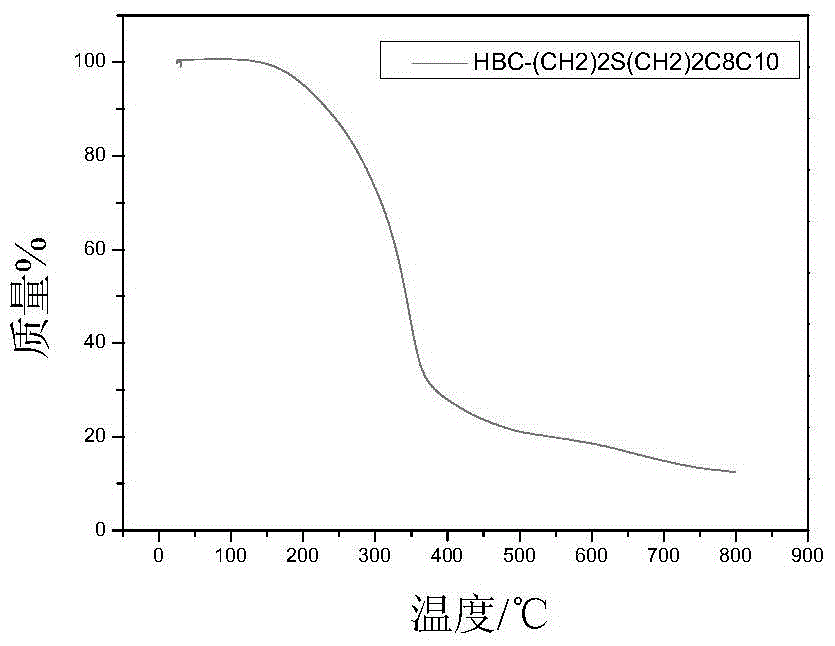
[0093] Embodiment 2: prepare HBC-(CH 2 ) 2 SCH 2 CH(C 8 h 17 )C 10 h 21
[0094] (1) Preparation of 4-bromo-phenethyl (2-octyl dodecyl) sulfide
[0095] Adopt p-bromophenethyl mercaptan (4.93g, 22.7mmol) prepared as embodiment 1 step (1), bromo 2-octyl dodecane (9.86g, 27.3mmol), method is the same as in embodiment 1 Step (2), the productive rate is 85%.
[0096] By nuclear magnetic resonance spectrum analysis, the product obtained is 4-bromo-phenethyl (2-octyl dodecyl) sulfide, and the specific data are: 1 HNMR (400MHz, CDCl 3 ):δ(ppm)δ0.86(t,3H,-CH 3 ),1.27(m,33H,-CH 2 -),2.47(d,2H,-CH 2 -),2.71(t,2H,-CH 2 -),2.81(t,2H,-CH 2 -), 7.07 (d, 2H, ArH), 7.40 (d, 2H, ArH).
[0097] (2) 1,2-bis((2-octyldodecyl)thioethylphenyl)acetylene (TOLAN-(CH 2 ) 2 S CH 2 CH(C 8 h 17 )C 10 h 21 )preparation
[0098] Using p-bromophenethyl(2-octyldodecyl)sulfide (4.14g, 8.3mmol), benzene (30ml), Pd(II) (dichlorobis(triphenylphosphine) palladium, 10% mol), CuI (cuprous iodi...
Embodiment 3
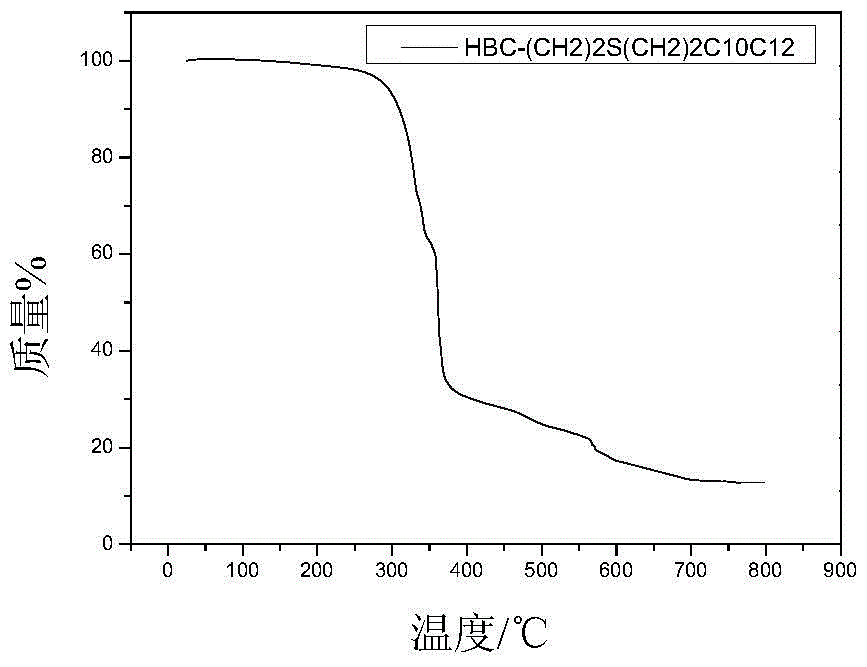
[0108] Embodiment 3: prepare HBC-(CH 2 ) 2 SCH 2 CH(C 10 h 21 )C 12 h 25
[0109] (1) Preparation of p-bromophenethyl (2-decyltetradecyl) sulfide
[0110] Adopt the p-bromophenethylmercaptan (9.77g, 45mmol) prepared as embodiment 1 step (1), bromo 2-decyltetradecane (20.55g, 49mmol), method is with the step in embodiment 1 ( 2), the yield is 82%.
[0111] By nuclear magnetic resonance spectrum analysis, the product obtained is p-bromophenethyl (2-decyltetradecyl) sulfide, and the specific data are 1 HNMR (300MHz, CDCl 3 ):δ(ppm)δ0.87(t,6H,-CH 3 ),1.26(m,41H,-CH 2 -),2.50(d,2H,-CH 2 -),2.72(t,2H,-CH 2 -),2.84(t,2H,-CH 2 -), 7.09 (d, 2H, ArH), 7.40 (d, 2H, ArH).
[0112] (2) 1,2-bis((2-decyltetradecyl)thioethylphenyl)acetylene (TOLAN-(CH 2 ) 2 S CH 2 CH(C 10 h 21 )C 12 h 25 preparation
[0113] Using p-bromophenethyl (2-decyltetradecyl) sulfide 5.54g, 10mmol), benzene (30ml), Pd (II) (dichloro bis (triphenylphosphine) palladium, (10%mol ), CuI (cuprous io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com