Medicine composition for anesthesia and sedation hypnosis and application of medicine composition for anesthesia and sedation hypnosis
An anesthesia hypnosis and composition technology, applied in the field of medicine, can solve problems such as social function impairment, influence on work and study, cognitive ability decline, etc., and achieve remarkable effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Effect test of QSK-02 on mouse non-alcoholic fatty liver model
[0022] 36 clean-grade Kunming mice, half male and half female, weighing (20±2) g, were randomly divided into blank control group, diazepam positive control group (2mg / kg), QSK-02 group (50mg / kg), Once a day, for 3 consecutive days, the volume of gavage was 20ml / kg, and the blank control group was gavaged with the same volume of distilled water. After 60 min of administration on the second day, the mouse autonomic activity meter was used to record the number of standing and the number of activities of the mice within 5 min after they were put into the box. After 60 minutes of the last administration, the mice in each group were intraperitoneally injected with pentobarbital sodium 50 mg / kg, and sleep was indexed by the disappearance of righting reflex, and those who kept the animal’s back-down posture for more than 30 seconds were judged as the disappearance of righting reflex. The time from the ...
Embodiment 2
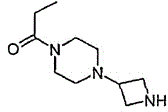
[0027] Embodiment 2: Preparation of 1-azetidin-3-yl-4-propionylpiperazine
[0028] (a) 1-[1-(Diphenylmethyl)azetidin-3-yl]piperazine
[0029] 1-(Diphenylmethyl)azetidin-3-yl methanesulfonate (see J.Org.Chem.; 56; 1991; 6729; 25 g, 78.6 mmol) was stirred under nitrogen at 60°C , piperazine (67.7 g, 0.79 mol) and a mixture of anhydrous acetonitrile overnight. The mixture was cooled and partitioned between water and dichloromethane. The organic layer was washed with water and brine. The solution was dried over Na2SO4, and the solvent was evaporated. The residue was purified by silica gel column chromatography (methanol-dichloromethane 5:95). 17.5 g (72%) of 1-[1-(diphenylmethyl)azetidin-3-yl]piperazine were obtained as a yellow oil. 1HNMR (400MHz, CDCl3): 2.1-2.4(m, 4H), 2.8-2.9(m, 2H), 3.0(m, 4H), 3.4-3.5(m, 2H), 3.7-3.9(m, 1H), 4.4 (s, 1H), 7.2-7.4 (m, 10H); LCMS: m / z 308 (M+1)+.
[0030] (b) 1-[1-(diphenylmethyl)azetidin-3-yl]-4-propionylpiperazine
[0031] 1-[1-(Diphe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com