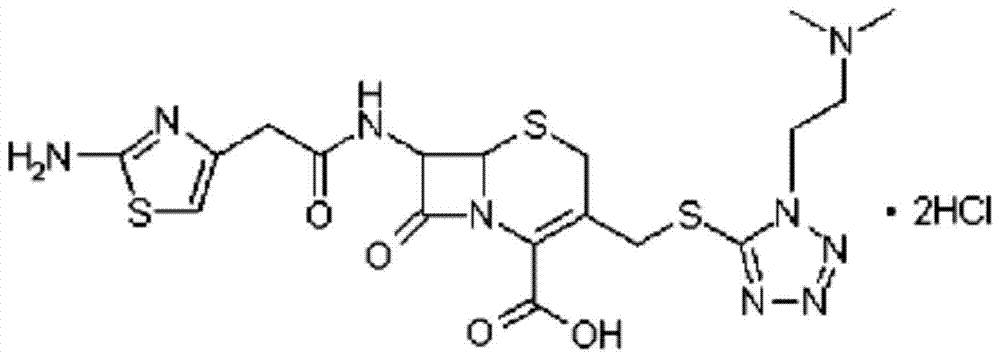
A kind of cefotiam hydrochloride compound, its preparation method and its pharmaceutical composition
A technology of cefotiam hydrochloride and cephalosporin hydrochloride, which is applied in the field of medicine, can solve the problems of poor mixing uniformity, poor fluidity of cefotiam hydrochloride, instability of cefotiam hydrochloride for injection, etc., and achieve good fluidity and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
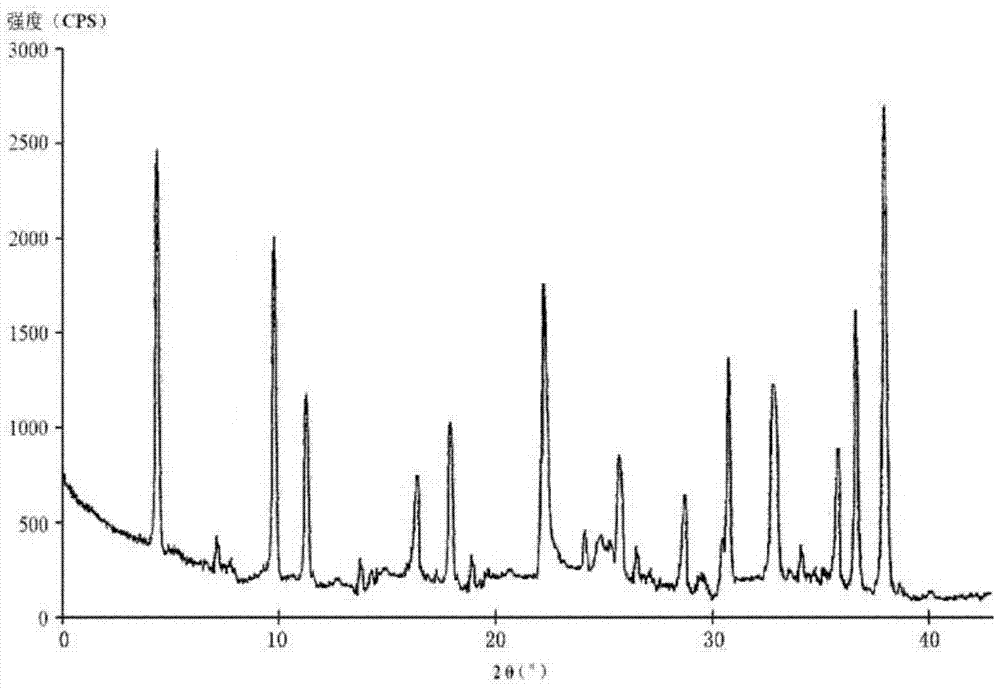
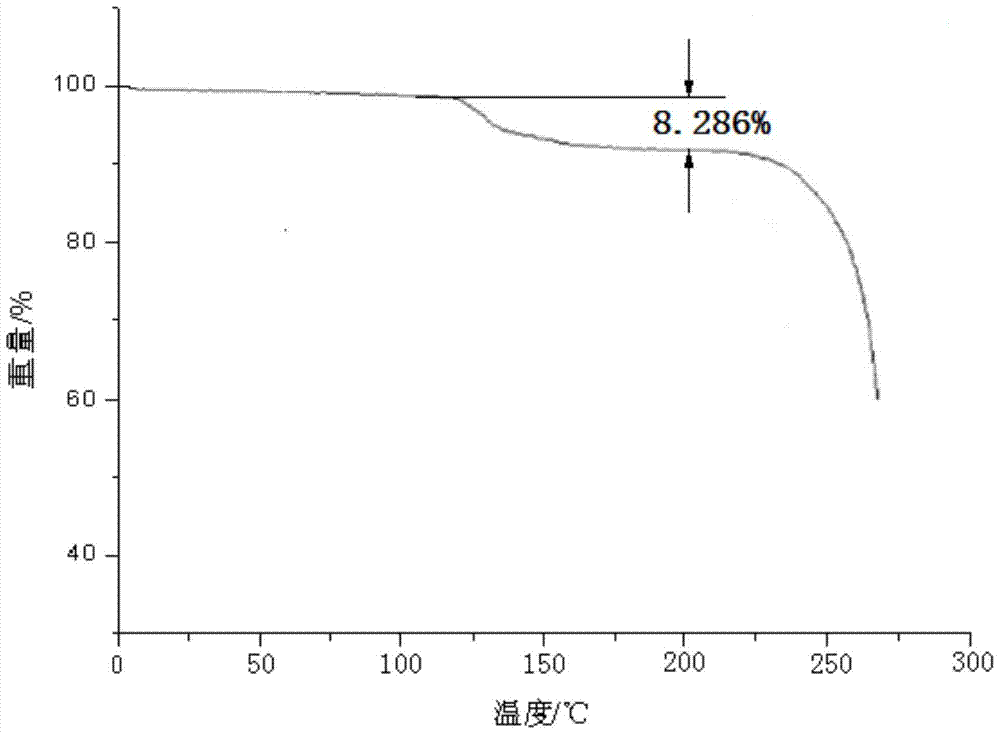
[0046] Embodiment 1, the preparation of cefotiam hydrochloride compound
[0047] [Example 1] Preparation of Cefotiam Hydrochloride Compound
[0048] 1) 12kg cefotiam hydrochloride crude product is dissolved in 10L water, obtains the cefotiam hydrochloride aqueous solution of 1.2kg / L;
[0049] 2) Add activated carbon (the amount of activated carbon is 0.3% g / ml of the volume of the cefotiam hydrochloride aqueous solution) to the cefotiam hydrochloride aqueous solution in step 1), stir, suction filter, and get the filtrate;
[0050] 3) Add 50L of organic solvent A (the flow rate of organic solvent A is 13L / min) to the filtrate of step 2) under the condition that the stirring speed is 50r / min to form a turbid solution, wherein said organic solvent A is ethanol A mixed solvent composed of dioxane and dioxane at a volume ratio of 2:1;
[0051] 4) After adjusting the stirring speed to 15r / min, add 300L of organic solvent B to the turbid solution of step 3) (the flow rate of organi...
Embodiment 2
[0057] [embodiment 2] the preparation of cefotiam hydrochloride compound
[0058] 1) 22kg cefotiam hydrochloride crude product is dissolved in 10L water, obtains the cefotiam hydrochloride aqueous solution of 2.2kg / L;
[0059] 2) Add activated carbon (the amount of activated carbon is 0.3% g / ml of the volume of the cefotiam hydrochloride aqueous solution) to the cefotiam hydrochloride aqueous solution in step 1), stir, suction filter, and get the filtrate;
[0060] 3) Add 100L organic solvent A (the flow rate of organic solvent A is 20L / min) to the filtrate of step 2) under the condition that the stirring speed is 60r / min to form a turbid solution, wherein the organic solvent A is ethanol A mixed solvent composed of dioxane and dioxane at a volume ratio of 3:1;
[0061] 4) After adjusting the stirring speed to 25r / min, add 200L of organic solvent B to the turbid solution of step 3) (the flow rate of organic solvent B is 6L / min). It is a mixed solvent composed of acetone and ...
Embodiment 3
[0066] [embodiment 3] the preparation of cefotiam hydrochloride compound
[0067] 1) 20kg cefotiam hydrochloride crude product is dissolved in 10L water, obtains the cefotiam hydrochloride aqueous solution of 2.0kg / L;
[0068] 2) Add activated carbon (the amount of activated carbon is 0.3% g / ml of the volume of the cefotiam hydrochloride aqueous solution) to the cefotiam hydrochloride aqueous solution in step 1), stir, suction filter, and get the filtrate;
[0069] 3) Add 80L of organic solvent A (the flow rate of organic solvent A is 15L / min) to the filtrate of step 2) under the condition that the stirring speed is 55r / min to form a turbid solution, wherein the organic solvent A is ethanol A mixed solvent composed of dioxane and dioxane at a volume ratio of 2.5:1;
[0070] 4) After adjusting the stirring speed to 20r / min, add 100L of organic solvent B to the turbid solution of step 3) (the flow rate of organic solvent B is 8L / min), after adding, crystals are precipitated, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com