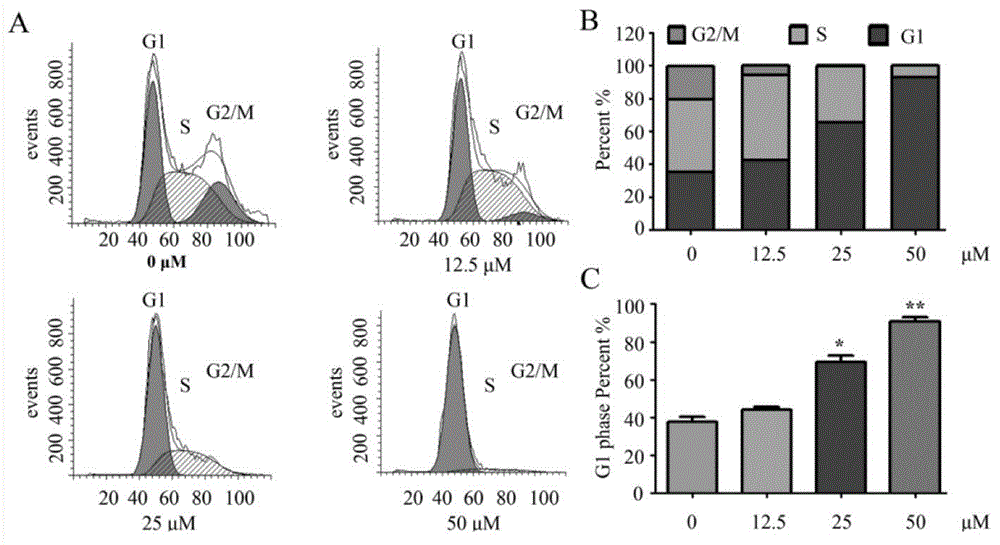
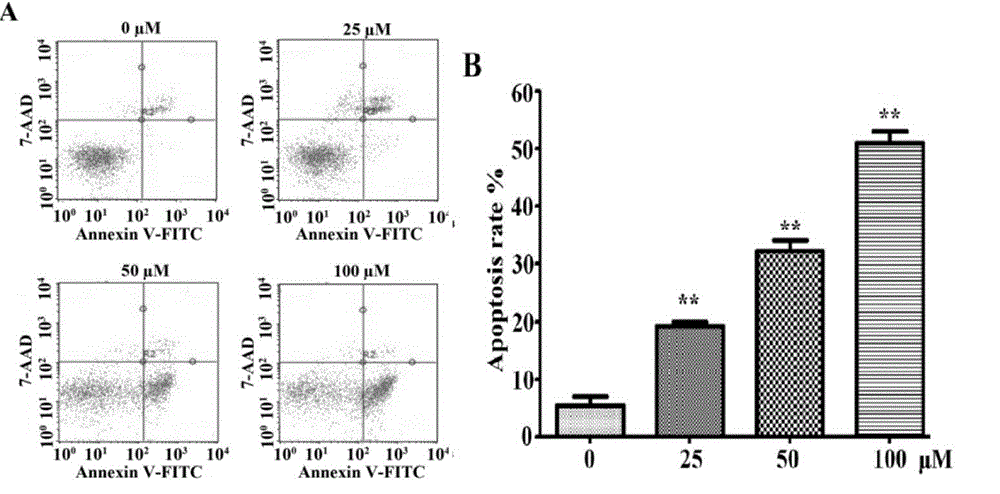
Matrine derivative and application thereof
A technology of matrine and derivatives, applied in the fields of benzodioxanes, benzene rings, naphthalene rings and indole matrine derivatives, and quinolines, can solve the problem of no antitumor activity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] YFF-1~YFF-9 in general formula I, YFF-10~YFF-15 in general formula II, and YFF-19 quinoline, benzene ring, benzodioxane, naphthalene ring, The preparation method of indole matrine derivatives.
[0030] (1) Preparation of compound YFF-1
[0031] Under nitrogen protection and ice bath conditions, 5 mmol (1.24 g) of matrine was dissolved in 20 mL of anhydrous tetrahydrofuran (THF), and 6 mmol (3 mL) of lithium diisopropylamide (LDA) was injected into In the reaction flask, after stirring and reacting at room temperature for 30 minutes, under ice cooling, add 10 mmol (1.89 g) ethyl indole-2-carboxylate into the flask with a syringe, and stir for 3 hours. Add 10 mL of saturated sodium chloride solution to quench the reaction, extract with chloroform (50 mL x 3), combine the organic phases, dry over anhydrous NaSO4, filter with suction, concentrate the filtrate, and purify by silica gel column chromatography (v methanol:v ethyl acetate Esters = 1:10), and 1.03g of white pow...
Embodiment 2
[0051] The preparation method of the YFF-16~YFF-18 naphthalene ring matrine derivatives in the general formula III.
[0052] (1) Preparation of compound YFF-16
[0053] Add 116 mmol (2.8 g) of sodium hydride and 50 mL of anhydrous tetrahydrofuran into a 100 mL round-bottomed flask, stir well, add 5 mmol (1.24 g) of matrine, slowly raise the temperature to 80°C and add 10 mmol (1.86 g)6 -Methoxy-2-naphthaldehyde, reacted to the end point (TLC detection). After cooling, adjust the reaction solution to neutrality with 3N hydrochloric acid. Extract with dichloromethane (20 mL×3), combine the organic phases, dry over anhydrous NaSO4, filter with suction, and concentrate the filtrate to obtain a yellow oil. Silica gel column chromatography (V methanol: V ethyl acetate = 1:10) purified to obtain 0.81 g of white solid 14-[2-(6-methoxy)naphthylmethylenyl]matrine with a yield of 39%.
[0054] (2) Compounds YFF-17~YFF-18 were prepared according to the method of compound YFF-16.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com