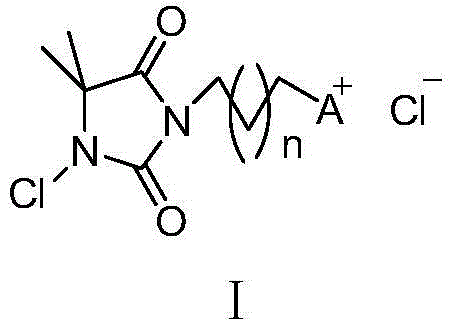
Quaternary phosphonium salt type N-halamine antibacterial agent and preparation method thereof
A technology of quaternary phosphonium salt and antibacterial agent, which is applied in the field of quaternary phosphonium salt type haloamine antibacterial agent molecule and its preparation, and can solve the problems of poor water solubility of haloamine antibacterial agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
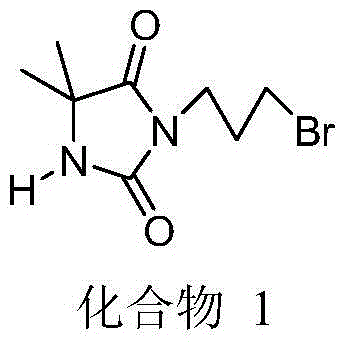
[0030] First add 4 g (about 31.2 mmol) of 5,5-dimethylhydantoin into a 500 mL single-necked flask, then add 200 mL of acetone, and place the flask in an oil bath to preheat. After the solid dissolves, add about 17g K 2 CO 3 (about 123.2mmol), add 40-50mL of acetone, reflux for 0.5h, add 18.9g of 1,3-dibromopropane (about 9.6mL, 93.7mmol) into the reactor, and continue to heat and reflux overnight. Wash with 100mL of water for several times, remove the inorganic salt impurities from the reacted mixture, and remove the solvent under vacuum to obtain a crude product. Column chromatography can further improve its purity, and the yield is about 85%. The product structure is shown in the following formula :
[0031]
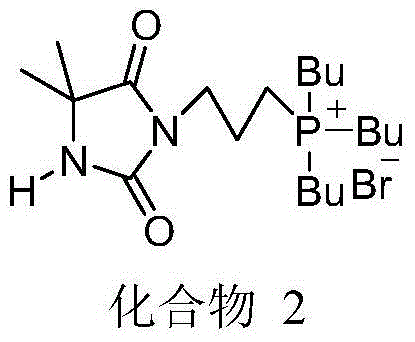
[0032] First 3.0g (about 12.1mmol) of compound 1 was dissolved in about 50mL of acetonitrile, and N was introduced into the reactor. 2 Exhaust the air, then inject 1.6g (about 2mL, 8.0mmol) of tributylphosphine into the reactor with a syringe, heat to reflux for ...
Embodiment 2
[0041] Dissolve 3.2 g (about 25.3 mmol) of 5,5-dimethylhydantoin in about 200 mL of acetone, and add K to the reactor after the dissolution is complete. 2 CO 313.8g (about 101.2mmol), heated to reflux for 0.5h, then added 24.9g (about 75.9mmol) of 1,12-dibromododecane into the reactor, continued to reflux for about 24h, then stopped the reaction. After removing the inorganic salt impurities, concentrate and remove the solvent under vacuum conditions, and then obtain compound 17 after purification by column chromatography, the yield is above 70%, and the structure is shown in the following formula:
[0042]
[0043] 2.5 g of compound 5 (about 6.7 mmol) was dissolved in 40 mL of acetonitrile. After the dissolution is complete, feed N into the reactor 2 Bleed out the air completely. Then inject 1.2 g of tributylphosphine (about 1.5 mL, 6.0 mmol) into the reactor, continue to heat and reflux for 48 h, and stop the reaction. The reaction solution was concentrated under vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com