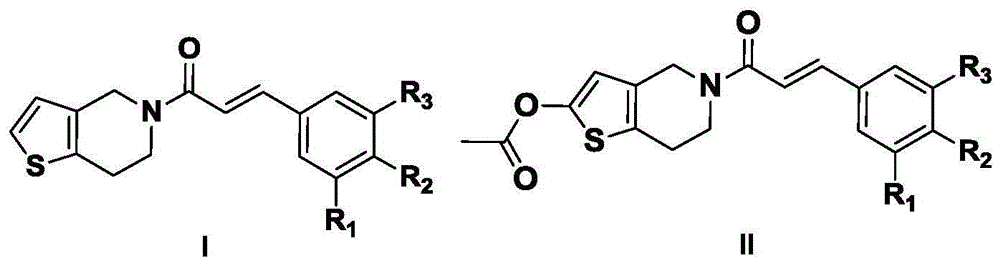
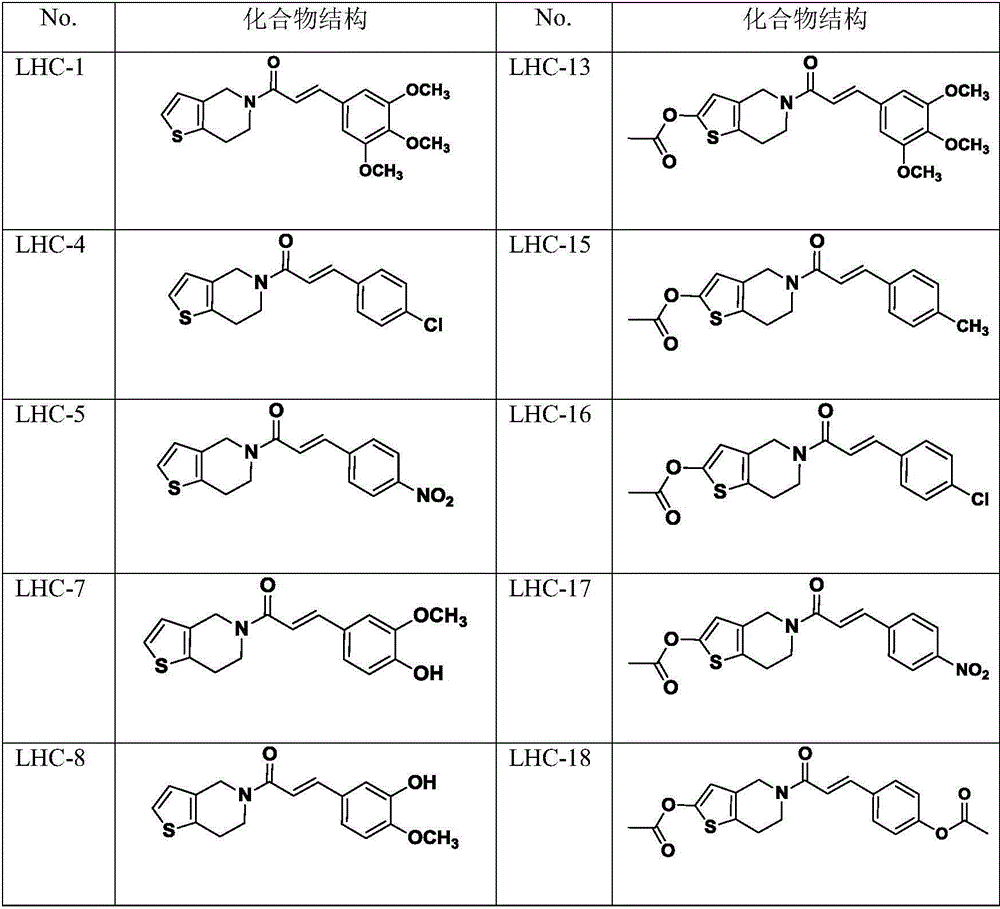
Perylene amide analog, preparation method and application thereof
A technology of perylene amide and analogs, applied in the field of medicinal chemistry, can solve the limitation of clinical application, large, can cause edema of adjacent renal tubules and glomerular epithelial cells and renal tubular hemorrhage, delayed hemorrhage "clopidogrel" and other issues, to achieve the effect of good inhibitory activity and good medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (E)-1-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-3-(3,4,5-trimethoxyphenyl)propan-2 Synthesis of -en-1-one (LHC-1)
[0031] 1.1 Synthesis of (E)-3-(3,4,5-trimethoxyphenyl)acrylic acid
[0032]
[0033] In a 500mL three-necked flask, add 3,4,5-trimethoxybenzaldehyde (10.0g, 51.0mmol), malonic acid (6.41g, 61.5mmol), pyridine (35mL), piperidine (2mL) and benzene (150mL), install an oil-water separator, and reflux at 110°C for 6h. TLC [V (chloroform): V (methanol) = 10:1 is the developing agent] detection shows that the reaction is almost complete, cool to room temperature, add 75 (mL) saturated aqueous sodium carbonate solution, continue to stir for 30 min, separate, take the water phase, the water phase Use 3 mol / L hydrochloric acid to adjust the pH to 4, and a large amount of white solid precipitates; suction filtration, recrystallization of the filter cake with absolute ethanol, and drying gives 7.92 g of white solid, yield 65.2%, m.p.124.3~125.7°C.
[0034] 1.2(...
Embodiment 2
[0039] (E)-1-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-3-(4-methoxyphenyl)prop-2-ene-1 -Synthesis of ketone (LHC-2)
[0040]
[0041] (E)-3-(4-methoxyphenyl)acrylic acid was synthesized according to the method 1.1 in Example 1, and (E)-1-(6,7-dihydrothiophene was obtained by operating according to the method 1.2 in Example 1 And[3,2-c]pyridin-5(4H)-yl)-3-(4-methoxyphenyl)prop-2-en-1-one (LHC-2) light yellow solid, m.p.131.4~ 132.7°C; 1 H-NMR (CDCl 3 ,400MHz)δ:7.67(d,J=15.2Hz,1H,ArCH=),7.50(d,J=8.0Hz,2H,ArH),7.15(d,J=5.2Hz,1H,ThH),6.90( d,J=8.4Hz,2H,ArH),6.83-6.79(m,2H,ThH and-CH=),4.76(s,2H,Py-CH 2 ),3.97(br s,2H,Py-CH 2 ),3.84(s,3H,OCH 3 ),2.94(br s,2H,Py-CH 2 ); 13 C-NMR (CDCl 3 ,100MHz)δ:166.3,160.9,142.6,132.3,129.4,127.9,125.2,124.5,123.5,114.9,114.2,55.3,45.9,43.3,26.0; IR(KBr,cm -1 )υ: 3004.3, 2962.5, 2905.6, 1649.8, 1593.0, 1506.3, 1437.5, 1249.2, 1165.5, 1060.8, 1027.9, 980.1, 896.4, 824.6, 716.9; ESI-Mass for C 17 h 17 NO 2 S:m / z(M + +H)300.13.
Embodiment 3
[0043] (E)-1-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-3-(4-methylphenyl)prop-2-en-1- Synthesis of Ketones (LHC-3)
[0044]
[0045] (E)-3-(4-methylphenyl)acrylic acid was synthesized according to the method 1.1 in Example 1, and (E)-1-(6,7-dihydrothieno) was obtained according to the method 1.2 in Example 1 [3,2-c]pyridin-5(4H)-yl)-3-(4-methylphenyl)prop-2-en-1-one (LHC-3) white solid, m.p.145.8~147.2℃; 1 H-NMR (CDCl 3 ,400MHz) δ:7.69(d,J=15.6Hz,1H,ArCH=),7.45(d,J=7.6Hz,2H,ArH),7.19(d,J=8.0Hz,2H,ArH),7.15( d,J=5.2Hz,1H,ThH),6.89(d,J=15.6Hz,1H,-CH=),6.83(d,J=4.8Hz,1H,ThH),4.77(s,2H,Py- CH 2 ),3.97(br s,2H,Py-CH 2 ),2.95(br s,2H,Py-CH 2 ),2.37(s,3H,CH 3 ); 13 C-NMR (CDCl 3 ,100MHz)δ:166.2,142.9.139.9,132.4,129.5,127.6,125.2,124.5,123.5,116.5,116.2,45.9,43.2,25.9,21.4; IR(KBr,cm -1 )υ: 3076.1, 2923.6, 2833.9, 1643.9, 1596.0, 1518.3, 1455.5, 1452.5, 1323.9, 1219.3, 1054.8, 1013.0, 968.1, 812.6, 737.9, 660.1; ESI-Mass for C 17 h 17 NOS:m / z(M + +H)284.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com