Irinotecan hydrochloride composite phospholipid composition, preparation method and applications thereof
A technology of irinotecan hydrochloride and Kang compound phospholipids, which is applied in the direction of drug combination, pharmaceutical formula, liposome delivery, etc., can solve the problems of easy leakage of drugs, liposome aggregation, poor liposome stability, etc., to reduce ATP Content, inhibitory function, enhanced effect of sensitive type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation of embodiment 1 irinotecan hydrochloride complex phospholipid composition:
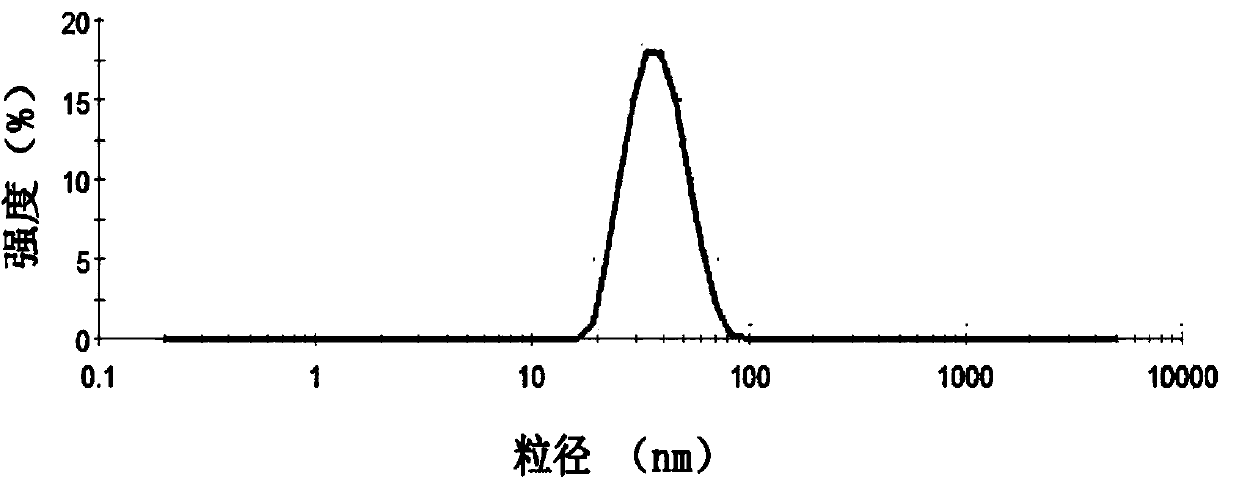
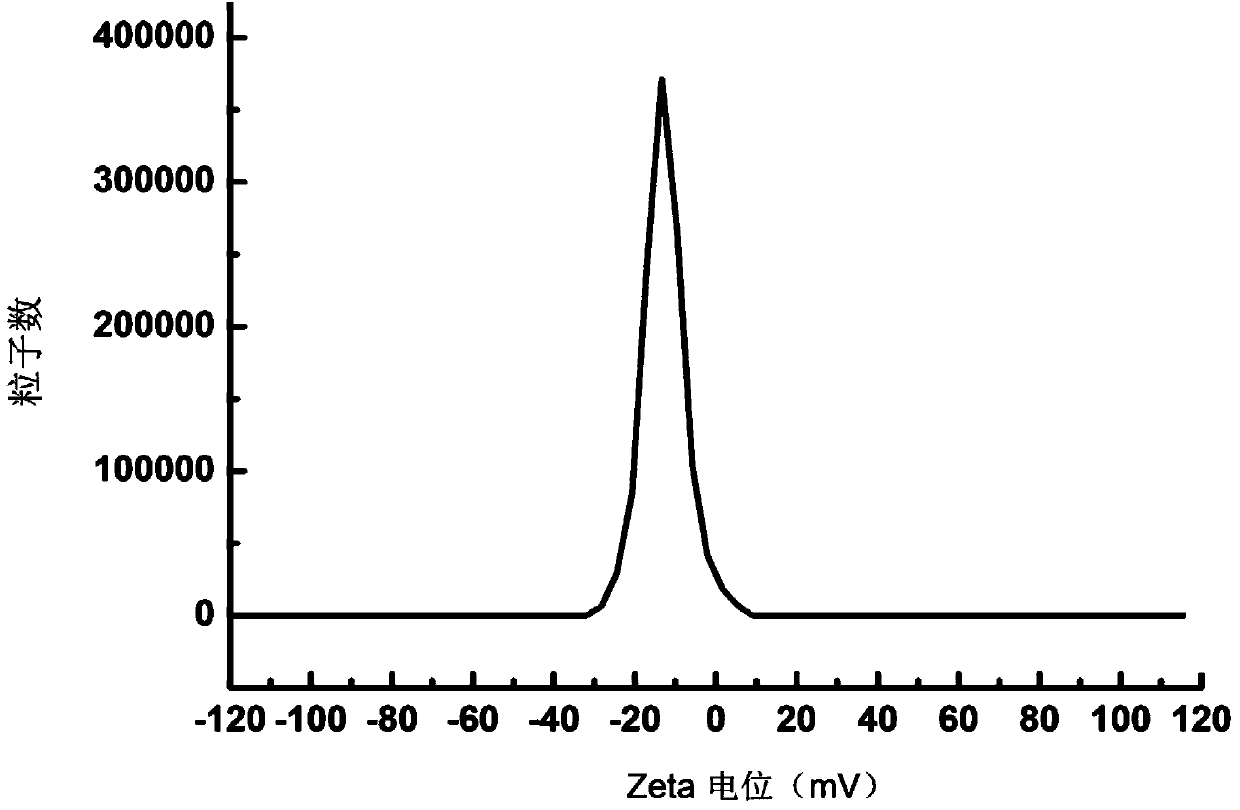
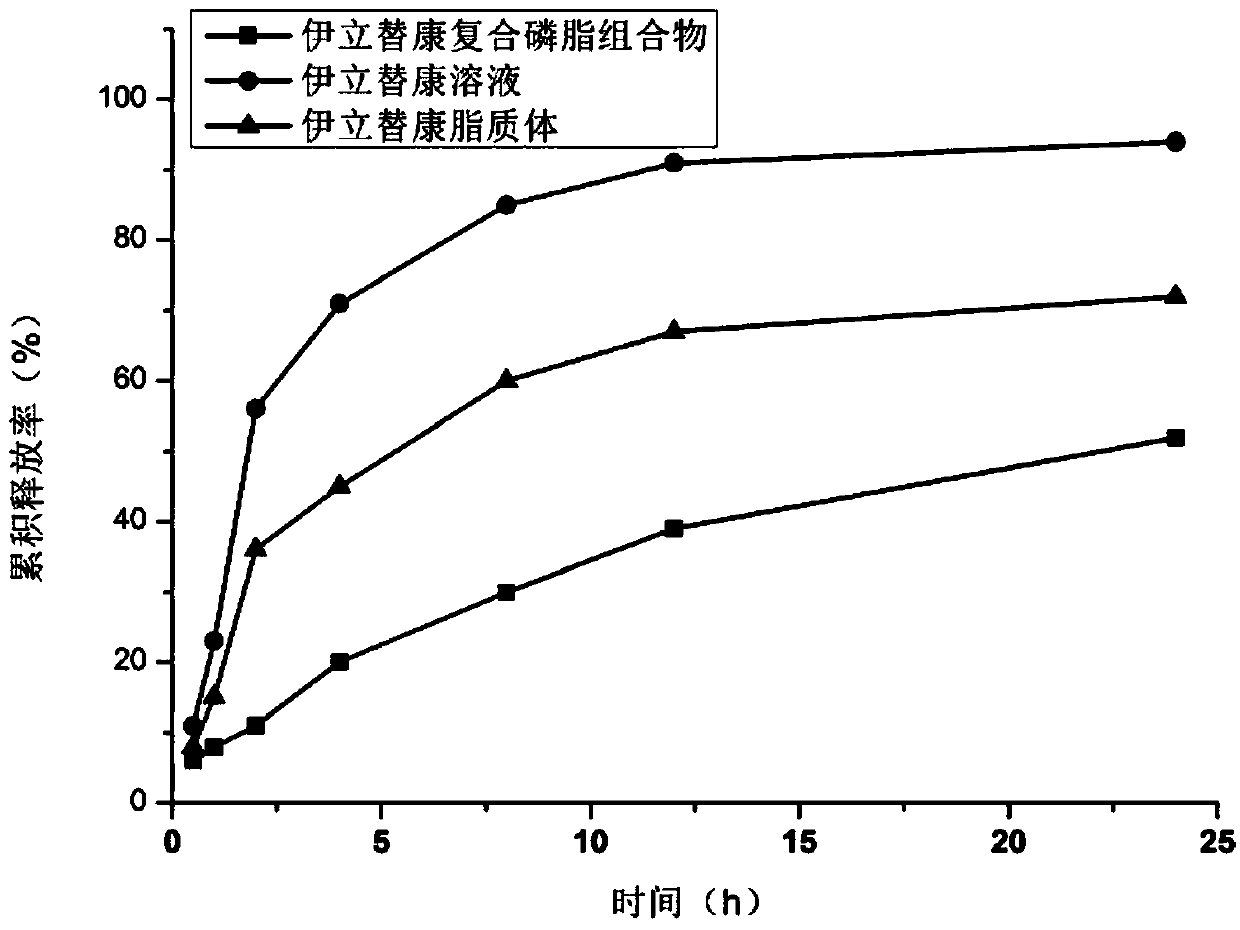
[0081] Dissolve 1.2g of HSPC, 0.012g of DSPC, 0.3g of cholesterol, and 0.4g of PEG2000-DSPE in 1.5ml of absolute ethanol by ultrasonication, inject it into 30ml of 250mM ammonium sulfate aqueous solution preheated to 65°C, and stir at high speed (20,000rpm) to obtain The first product was homogenized under high pressure for 4 times at 20000psi, and 10 times the volume of ammonium sulfate in the excepted aqueous phase was replaced with ultrapure water through a tangential flow ultrafiltration system (membrane molecular weight 30kDa, flow rate 200ml / min, pressure 1bar), namely Obtain a blank liposome suspension; mix the obtained blank liposome suspension with an aqueous solution of irinotecan hydrochloride (concentration: 10 mg / ml) according to the weight ratio of drug to HSPC at 1:10, incubate at 65°C for 30 min, and then add 0.012 g of F68 , 4.3g sucrose, 0.065g histidine, and a...
Embodiment 2
[0082] The preparation of embodiment 2 irinotecan hydrochloride complex phospholipid composition:
[0083] Weigh 1.5g of HSPC, 0.02g of soybean lecithin, 0.15g of cholesterol, and 0.15g of PEG2000-DSPE, dissolve them in 1.5ml of absolute ethanol, and inject them into 30ml of 200mM ammonium sulfate aqueous solution preheated to 65°C at high speed (25,000rpm) Stir to obtain the primary product, then homogenize under high pressure for 4 times at 15000psi, and replace 20 times the volume of ammonium sulfate except the aqueous phase with 300mM sucrose aqueous solution through a tangential flow ultrafiltration system (membrane molecular weight 30kDa, flow rate 300ml / min, pressure 1.5bar) , to obtain a blank liposome suspension. The resulting blank liposome suspension was mixed with irinotecan hydrochloride solution (concentration: 10mg / ml) at a weight ratio of drug to HSPC of 1:20, incubated at 55°C for 1 hour, and then passed through a tangential flow ultrafiltration system to remo...
Embodiment 3
[0084] The preparation of embodiment 3 irinotecan hydrochloride complex phospholipid composition
[0085] Weigh 1.2g of HSPC, 0.024g of egg yolk lecithin, 0.12g of cholesterol, and 0.4g of PEG2000-DPPE; ) stirred to obtain the primary product, and then extruded 4 times with a polycarbonate membrane with a pore size of 100nm, and replaced 5 times the volume with a 300mM sucrose aqueous solution through a tangential flow ultrafiltration system (membrane molecular weight 30kDa, flow rate 100ml / min, pressure 1.5bar) To remove the ammonium sulfate in the external aqueous phase, a blank liposome suspension was obtained. The resulting blank liposome suspension was mixed with irinotecan hydrochloride aqueous solution (concentration: 5 mg / ml) according to the weight ratio of drug to HSPC at 1:5, incubated at 60°C for 10 minutes, and then passed through a tangential flow ultrafiltration system to remove unencapsulated cells. The drug, and its external water phase was replaced with 300m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com