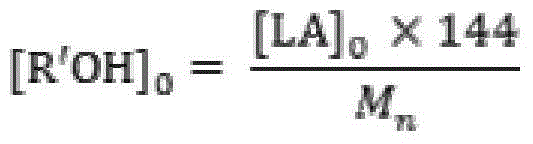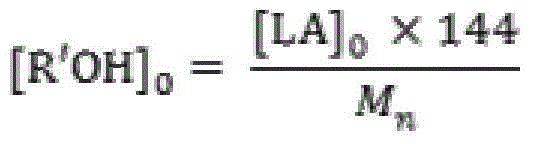Technology for controlled synthesis of polylactic acid through lactide activity ring-opening polymerization under catalytic action of organic guanidine-nontoxic alcohol
A technology for alcohol-catalyzed lactide and ring-opening polymerization, which is applied in the field of biodegradable polylactic acid to achieve the effects of high catalytic activity, high biosafety and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
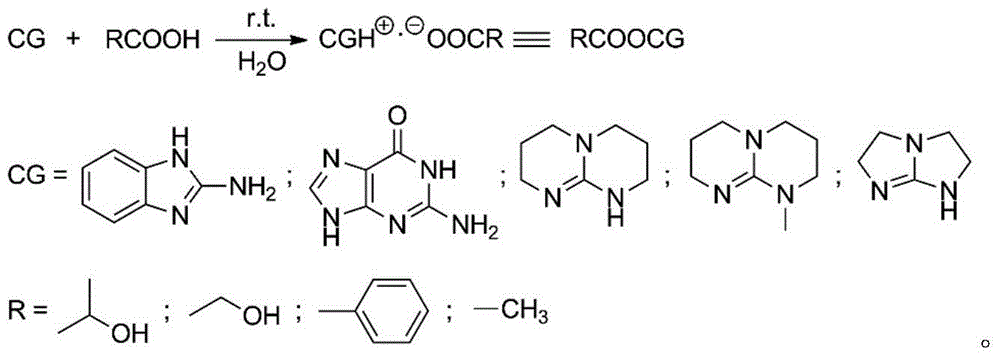
[0023] Add 50.0g (0.347mol) of monomer LLA, 0.048g (0.231mmol) of catalyst 2-aminobenzimidazole glycolate and 0.461g (10.000mmol) of initiator ethanol into the polymerization reactor, and pass through three times of "vacuumizing-nitrogen Circular operation to drive out the air in the polymerization kettle, seal the reaction kettle after the pressure in the kettle is constant at 1.0 torr, raise the temperature to 96°C within 30min with stirring, and then react at 130±1°C for 5min. Product PLLA: M n 0.5×10 4 , PDI 1.14, monomer conversion rate 100%, white color.
Embodiment 2
[0025] 50.0g (0.347mol) of monomer DLA, 0.060g (0.231mmol) of catalyst 1,5,7-triazabicyclo[5.5.0]dec-5-ene benzoate and 0.230g (5.000mmol) of initiator ethanol mmol) was added to the polymerization reactor, and the air in the polymerization reactor was removed through three "vacuumizing-nitrogen filling" cycle operations. After the pressure in the reactor was constant at 1.0 torr, the reactor was sealed, and the temperature was raised to 96 ° C within 30 minutes under stirring, and then React at 125±1°C for 30 minutes. Product PLLA: M n 1.0×10 4 , PDI 1.15, monomer conversion rate 100%, white color.
Embodiment 3
[0027] The monomer DLLA 100.0g (0.694mol), the catalyst 2,3,5,6-tetrahydro-1H-imidazo[1,2-A]imidazole lactate 0.095g (0.463mmol) and the initiator ethanol 0.307g (6.667mmol) was added to the polymerization reactor, and the air in the polymerization reactor was removed by three "vacuumizing-nitrogen filling" cycle operations. After the pressure in the reactor was constant at 0.6torr, the reactor was sealed, and the temperature was raised to 96°C within 30min under stirring. Then react at 118±1°C for 60 minutes. Product PLLA: M n 1.5×10 4 , PDI 1.10, monomer conversion rate 100%, white color.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com