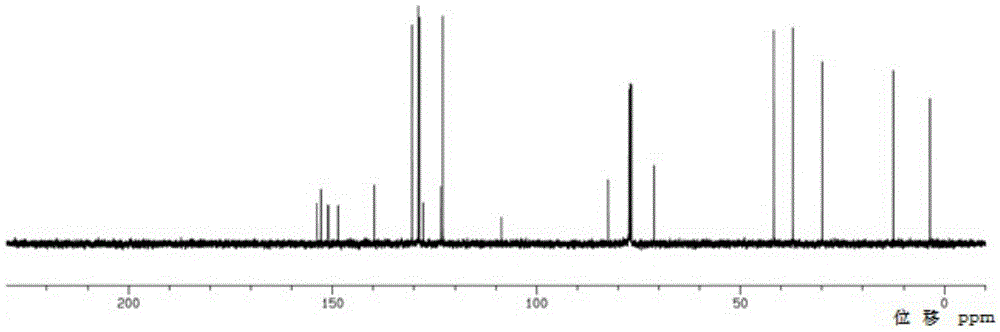
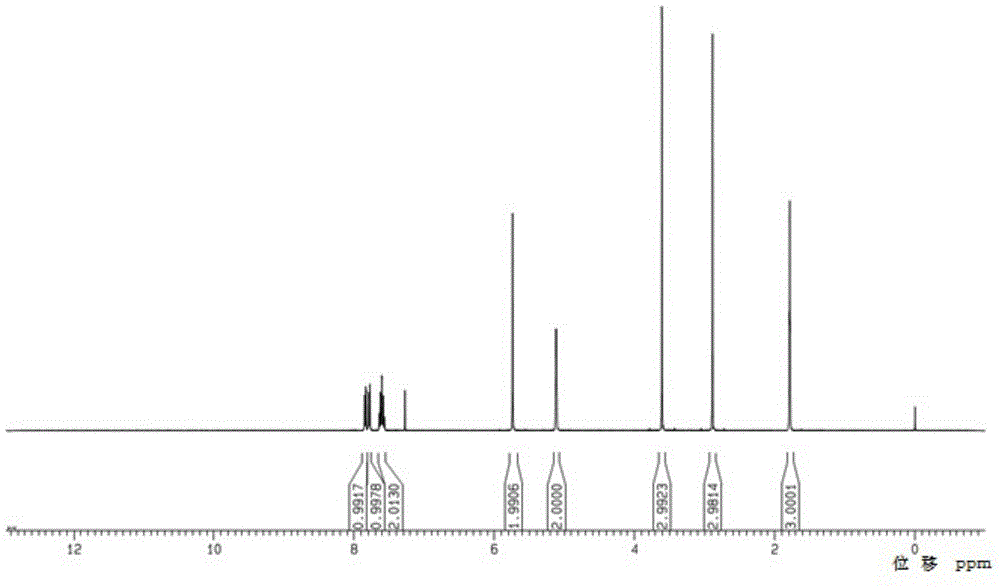
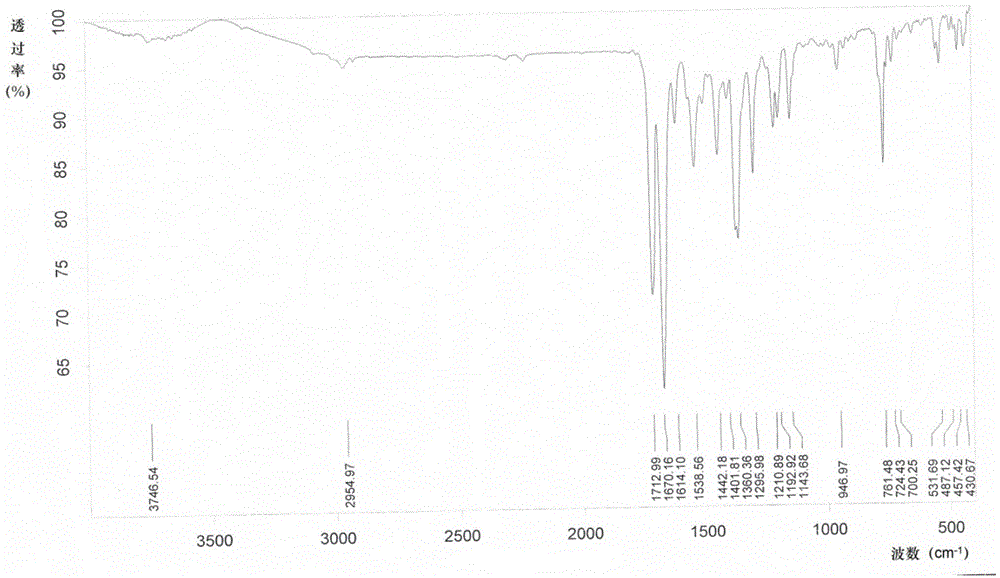
Linagliptin intermediate, preparation method and applications thereof
A reaction and complete reaction technology, applied in the field of medicine, can solve problems such as difficulties in industrialized large-scale production, many impurities, and large energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Synthesis of the compound shown in embodiment 1 formula (Ⅵ)
[0114]
[0115] Add 27g (0.2mol) of o-aminoacetophenone, 13.9g (0.2mol) of hydroxylamine hydrochloride, 240mL of absolute ethanol, and reflux at 80°C to monitor the reaction by HPLC; then Cool to 20°C, add 10% sodium hydroxide solution dropwise, adjust the pH of the reaction solution to 7.5, then cool the feed solution to 5°C, add an appropriate amount of water to make the reaction solution turbid, and let stand; then add about 400mL of water, grow crystals at 5°C for 30 minutes; filter with suction, wash with ethanol-water system, and dry in vacuo to obtain 24.84g of the compound shown in white solid formula (Ⅵ), with a molar yield of 82.8%, HPLC (%): 98.4% .
Embodiment 2
[0116] Synthesis of the compound shown in embodiment 2 formula (Ⅵ)
[0117]
[0118] Add 40.5g (0.3mol) of o-aminoacetophenone, 27.73g (0.399mol) of hydroxylamine hydrochloride, 360mL of anhydrous methanol, and reflux at 65°C to a 500mL four-necked flask with a thermometer, and monitor the reaction by HPLC; Then cool to 25°C, add 10% sodium carbonate solution dropwise, adjust the pH of the above liquid to 8.0, then cool the feed liquid to 15°C, add an appropriate amount of water to make the reaction liquid turbid, and let it stand; then add about 720mL dropwise water, grow crystals at 5-10° C. for 60 minutes; suction filtration, washing with methanol-water system, and vacuum drying to obtain about 37.04 g of the compound shown in white solid formula (Ⅵ), with a molar yield of 82.3%, HPLC (%): 98.1%.
Embodiment 3
[0119] Synthesis of the compound shown in embodiment 3 formula (Ⅵ)
[0120]
[0121] Add 13.5g (0.1mol) of o-aminoacetophenone, 13.9g (0.2mol) of hydroxylamine hydrochloride, 120mL of isopropanol, and reflux at 100°C to a 500mL four-necked flask with a thermometer, and monitor the reaction by HPLC; Then cool to 20°C, add 10% sodium hydroxide solution dropwise, adjust the pH of the reaction solution to 7.5, then cool the feed solution to 10°C, add an appropriate amount of water to make the reaction solution turbid, and let stand; then add About 260mL of water, then grow crystals at 5°C for 45 minutes; filter with suction, wash with isopropanol-water system, and dry in vacuo to obtain about 12.2g of the compound shown in white solid formula (Ⅵ), with a molar yield of 81.3%, HPLC ( %): 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com