A method to produce 2‑ chloric acid
A technology of chloronicotinic acid and chloronicotinic acid ethyl ester, which is applied in the direction of organic chemistry, can solve environmental hazards and other problems, and achieve the effects of environmental friendliness, significant economic and social benefits, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
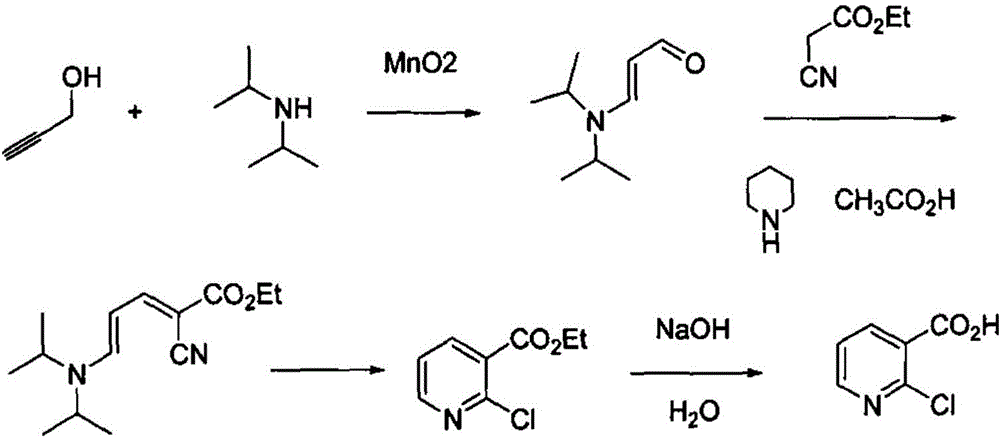
[0026] A method for producing 2-chloronicotinic acid, which comprises catalytic oxidation of propynyl alcohol and diisopropylamine to obtain diisopropylaminoacrolein, and then reacts with ethyl cyanoacetate to generate 2-cyano-5-diisopropylamine Base-2,4-pentadienoic acid ethyl ester, pass through dry hydrogen chloride, will obtain 2-chloronicotinic acid ethyl ester through alkaline hydrolysis, after acidification, obtain 2-chloronicotinic acid, the reaction is as follows:
[0027]
[0028] Preparation of Diisopropylaminoacrolein
[0029] 51 g of diisopropylamine 51 g, 28 g of propynyl alcohol, 2.5 g of manganese dioxide and 200 mL of toluene were sequentially added to a 0.5 L autoclave, and the reactor was closed. After raising the temperature to 80°C, feed oxygen, keep the internal pressure of the reactor at 0.1-0.2Mpa, and keep warm for 8-10h. After testing that the reaction of the raw materials is complete, filter the reaction solution, wash the filter cake with a smal...
Embodiment 2
[0037] A method for producing 2-chloronicotinic acid, adopting the following steps:
[0038] 1) propynyl alcohol and diisopropylamine are mixed and dissolved in toluene at a molar ratio of 1:1, manganese dioxide is added as a catalyst, air is passed through, and the reaction is carried out at 30°C and 2Mpa for 20 hours, the catalyst is removed by filtration, and methanol is used as solvent, and the filtrate is precipitated to obtain diisopropylamino acrolein;
[0039] 2) After dissolving diisopropylaminoacrolein in toluene, add methyl cyanoacetate and catalyst triethylamine, the molar ratio of diisopropylaminoacrolein to cyanoacetate is 1:0.95 and reflux for 6 hours, using ethanol As a solvent, get 2-cyano-5-diisopropylamino-2,4-pentadienoate through precipitation;
[0040] 3) Dissolve ethyl 2-cyano-5-diisopropylamino-2,4-pentadienoate in ethanol, pass through dry hydrogen chloride gas, the pressure of hydrogen chloride gas is 0.8Mpa, and then under the conditions of 5°C and ...
Embodiment 3
[0043] A method for producing 2-chloronicotinic acid, adopting the following steps:
[0044] 1) propynyl alcohol and diisopropylamine are mixed and dissolved in toluene at a molar ratio of 1:1, manganese dioxide is added as a catalyst, oxygen is passed through, at 60°C and 1Mpa, react for 12 hours, filter to remove the catalyst, and use toluene as solvent, and the filtrate is precipitated to obtain diisopropylamino acrolein;
[0045] 2) After dissolving diisopropylaminoacrolein in toluene, add ethyl cyanoacetate and catalyst sodium carbonate, the molar ratio of diisopropylaminoacrolein to cyanoacetate is 1:0.9 and reflux for 8 hours, using toluene as Solvent, precipitation to obtain 2-cyano-5-diisopropylamino-2,4-pentadienoate;
[0046]3) Dissolve ethyl 2-cyano-5-diisopropylamino-2,4-pentadienoate in ethanol, pass through dry hydrogen chloride gas, the pressure of hydrogen chloride gas is 1.0Mpa, and react at 20°C and 1Mpa for 18 Hours, with toluene as solvent, the oil phase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com