Method and apparatus for simulating blood flow under patient-specific boundary conditions derived from an estimated cardiac ejection output
A technology of cardiac ejection and boundary conditions, applied in blood flow measurement devices, blood flow measurement, and instruments for radiological diagnosis, etc., can solve problems such as unclear pressure gradients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
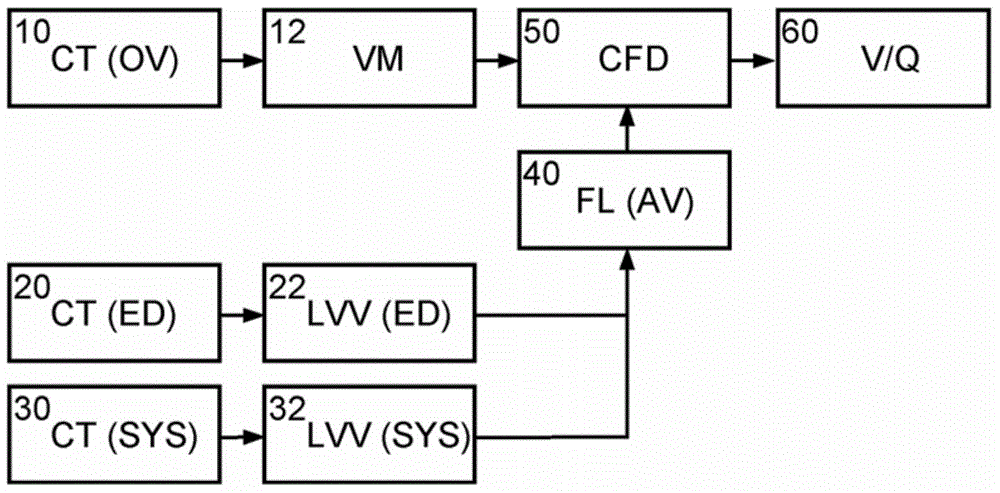
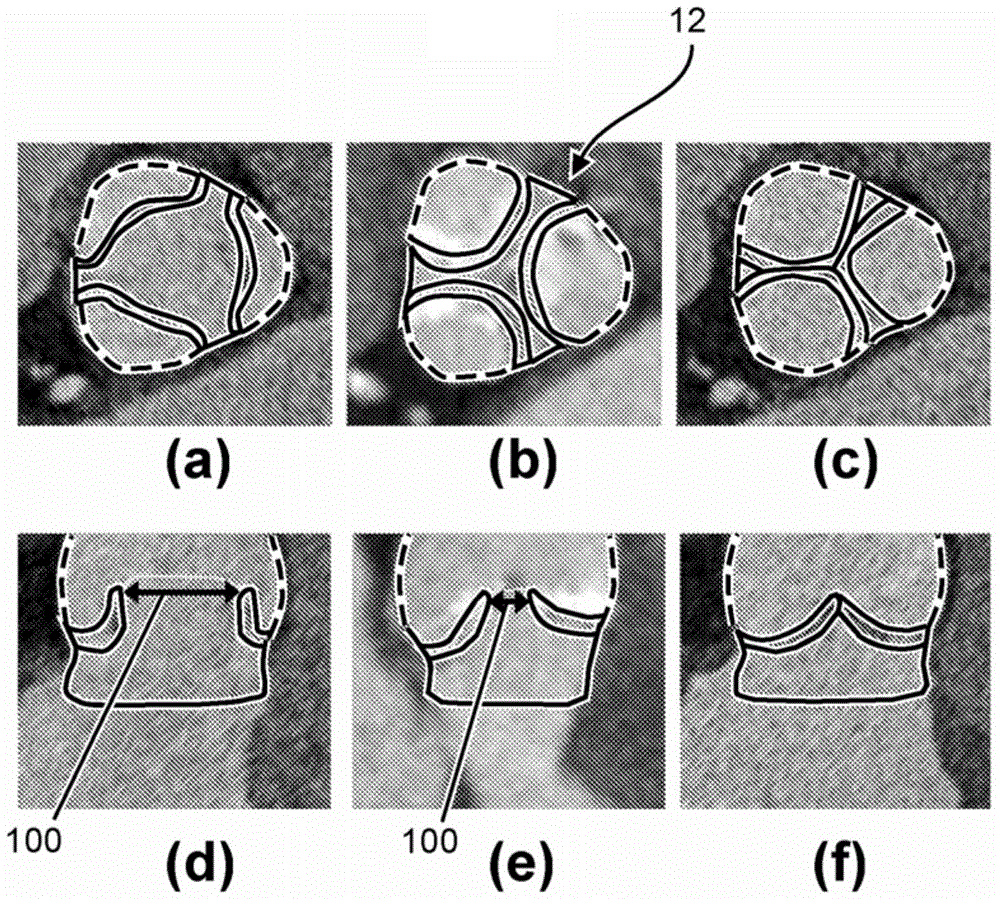
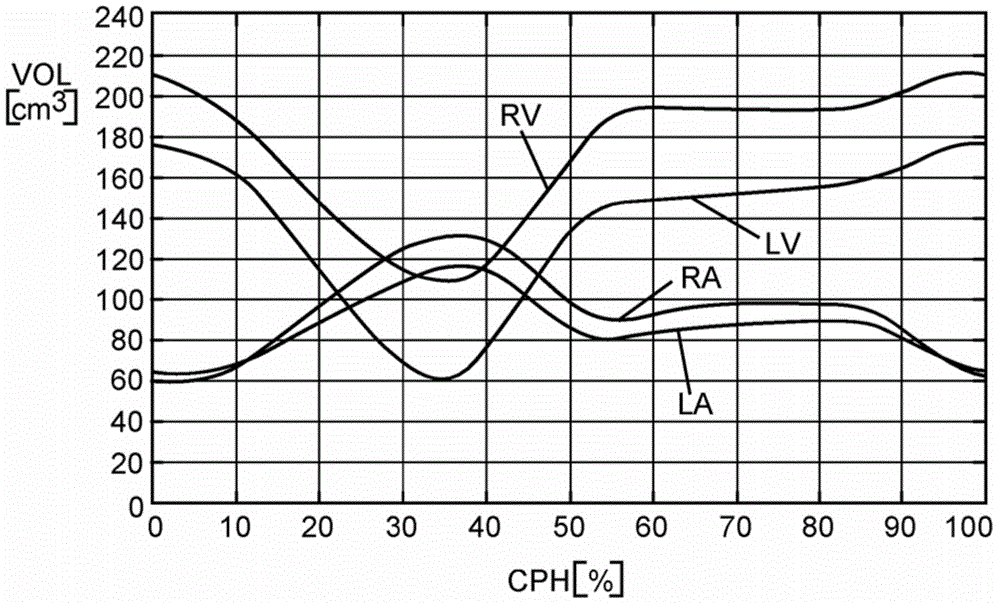
[0026] Now based on the addition of the ascending aorta through the left ventricular (LV) outflow tract (as an example of a blood cavity or cardiovascular structure close to the heart) under patient-specific boundary conditions derived from cardiac ejection output per heartbeat Embodiments are described for the simulation of blood flow in patient-specific geometries that can be derived from (at least) two images of the LV at states of maximum and minimum filling (e.g., end-diastole, end-systole) calculate. The geometry of the LV outflow tract, including the aortic root of the AV, plus ascending aorta and ventricular volumes can be obtained automatically by model-based segmentation.
[0027] figure 1 A schematic block diagram illustrating the generation and use of patient-specific boundary conditions for simulating flow through the aortic valve is shown. figure 1 The blocks can be regarded as hardware circuits adapted to perform the corresponding functions or as steps of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com