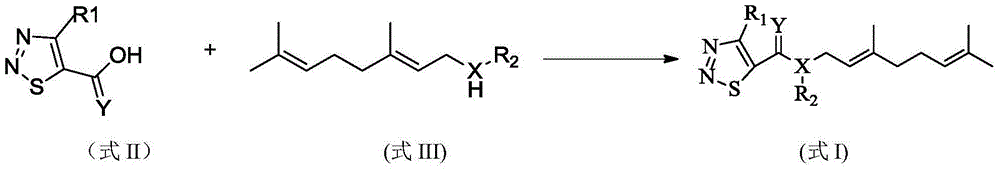
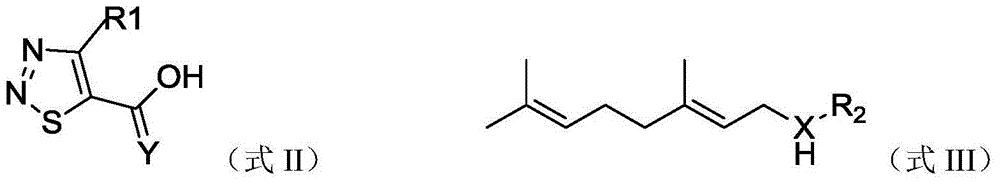
(cis)-beta-farnesene analog containing 1,2,3-thiadiazole group, preparation method, and applications thereof
A thiadiazole-based and farnesene technology, which is used in plant anti-virus, pest control, 1,2,3-thiadiazole group-β-farnesene analogs and their preparation, and applications in sterilization It can solve the problems of restricting field aphid control, poor stability, easy volatilization, etc., and achieve the effects of simple and easy preparation method, improved stability and large molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: (E)-3,7-dimethyl-2,6-octadienyl-(4-methyl-1,2,3thiadiazole)-5-carboxylate (No. 1) preparation of
[0031]Weigh 1.01g (7.0mmol) of 4-methyl-1,2,3-thiadiazole-5-carboxylic acid and 0.1g of DMAP into a 50ml three-neck flask, add 20ml of acetonitrile and stir to dissolve in an ice bath, batch by batch Add 1.57g (7.7mmol) DCC, then add 1.08g (7.0mol) geraniol to the system, react at room temperature, and detect the reaction by TLC. After the reaction is complete, filter, wash the mother liquor with saturated sodium bicarbonate solution and saturated sodium chloride solution, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography [V (ethyl acetate): V (petroleum ether) = 30:1 ], to obtain 1.24g of light yellow liquid, which is the compound 1 provided by the present invention, with a yield of 63.3%.
[0032]
[0033] According to exactly the same method as preparing compound 1, only the compound shown in formula II and R of the co...
Embodiment 2
[0043] Embodiment 2: The insecticidal activity of the compound of the present invention to aphids
[0044] Weigh 50mg of the target compound sample in a 20ml weighing bottle with a ten-thousandth balance, and introduce it into a 10mL volumetric flask to prepare a 5000mg / L measuring solution. Then use a 1-5ml pipette gun to take 1ml of acetone into a weighing bottle, add 9ml of an aqueous solution containing 0.1% Triton X-100, and mix well to obtain a 500mg / L measuring solution. Soybean leaves cultivated indoors that have not been exposed to any pesticides and insects are punched out with a 15mm diameter hole puncher, soaked in the diluted medicinal solution for 15 seconds, taken out to dry, and placed in a bioassay plate. With the back facing up, 1% agar was added to the bottom to keep it moist, and 20 soybean aphids were inserted into each well, and each was repeated 3 times. Check the results after 48 hours. The criteria for judging the death are: touch the insect body lig...
Embodiment 3
[0052] Embodiment 3: The repellent activity of the compound of the present invention to aphids
[0053] More than 20 green peach aphids were released from the release port, and humid air passed through activated carbon and distilled water was introduced into each arm through an air pump at 0.2 L / min. The moist air introduced by the test arm first passes through 5 μg of the sample odor source, and the other arm is used as the control arm, and the introduced moist air first passes through the solvent. Record the number of aphids in each arm when the sample was introduced for 15 minutes. For each repetition, the olfactometer and leather tube were cleaned with absolute ethanol, the filter paper was replaced, and the two arms were used interchangeably. The experiment of each sample was repeated four times. The aphids that crossed the center of the olfactometer by 2cm were included in the treatment group or the control group, and the aphids that did not cross were recorded as the u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com