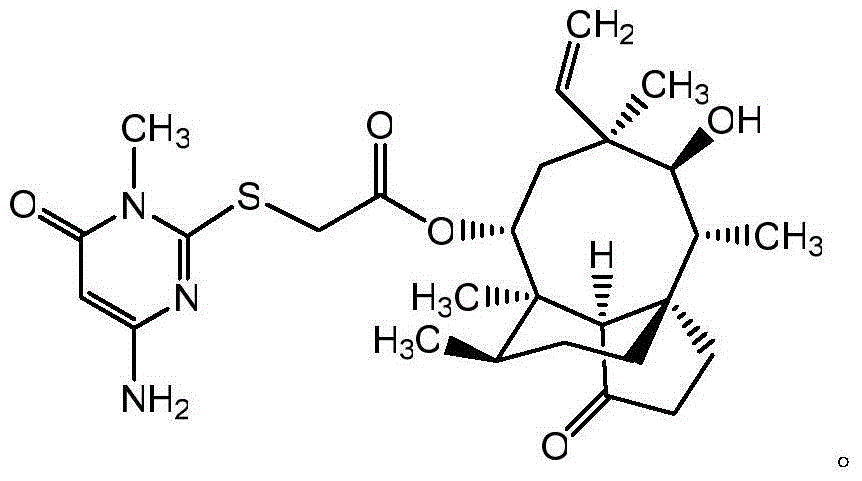
Pleuromutilin derivative with pyrimidine side chain and application of pleuromutilin derivative
A technology of pleuromutilin and derivatives, applied in the direction of organic chemistry, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: 14-O-[(4-Amino-6-hydroxy-pyrimidin-2-yl)thioacetyl]Multiline
[0021] Dissolve 1.65 g of 4-amino-6-hydroxy-2-mercaptopyrimidine monohydrate (10 mmol) in 20 mL of methanol, add 1.1 mL of 10 M NaOH (11 mmol) and stir for 30 min. Then 20 mL of dichloromethane (DCM) solution containing 5.33 g (10 mmol) 22-O-(4-tosyl)oxyacetylmtilin was added dropwise, and stirred at room temperature for 36-40 h after the addition was complete. After evaporating the solvent under reduced pressure, the crude product was dissolved in a mixed solution of 60mL ethyl acetate and 20mL water, and 50mL saturated NaHCO 3 Afterwards, a large amount of white precipitate was produced, and after filtration, it was separated by column chromatography (ethyl acetate:ethanol=20:1) to obtain the target compound.
[0022] Due to the structural tautomerism of the hydroxyl and ketone formulas, the 14-O-[(4-amino-6-hydroxy-pyrimidin-2-yl)thioacetyl]muroline tautomer is 14-O-[(4 -Amino-6-ketone-pyrimi...
Embodiment 2
[0028] Example 2: 14-O-[(4-Amino-6-methoxy-pyrimidin-2-yl)thioacetyl]Multiline
[0029] 2.51g of 14-O-[(4-amino-6-hydroxy-pyrimidin-2-yl) thioacetyl] monoclonal (5.0mmol) and 2.07g of K 2 CO 3 (15 mmol) was dissolved in 20 mL of dimethylformamide (DMF), then 0.71 g of iodomethane (5.0 mmol) was added in portions. Stir the reaction at 80-85°C for 4h, cool the reaction system, filter the insoluble impurities, and evaporate the solvent to dryness under reduced pressure. The resulting mixture was separated by column chromatography (petroleum ether: ethyl acetate = 1:15) to obtain 1.7 g (28%) of 14-O-[(4-amino-6methoxy-pyrimidin-2-yl ) thioacetyl]Miltiline and 3.6 g (61%) of 14-O-[(1-methyl-4-amino-6-keto-pyrimidin-2-yl)thioacetyl]Miltiline.
[0030] 14-O-[(4-Amino-6-methoxy-pyrimidin-2-yl)thioacetyl]Multiline, the structural formula is as follows:
[0031]
[0032]IR(KBr)3374,2928,1732,1626,1585,1548,1390,1307,1273,1212,1152,1117,1048,1017,982,917cm -1 ; 1 H NMR (400MHz, ...
Embodiment 3
[0033] Example 3: 14-O-[(1-Methyl-4-amino-6-keto-pyrimidin-2-yl)thioacetyl]Multiline
[0034] The preparation method of 14-O-[(1-methyl-4-amino-6-keto-pyrimidin-2-yl)mercaptoyl]Multiline is the same as in Example 2, and the structural formula is as follows:
[0035]
[0036] IR (KBr) 3422, 2929, 1730, 1630, 1509, 1457, 1414, 1282, 1154, 1117, 1094, 807; 1 H NMR (400MHz, DMSO) δ6.28(s, 2H), 6.10(dd, J=17.8, 11.2Hz, 1H), 5.52(d, J=8.1Hz, 1H), 5.05(dd, J=27.5, 14.5Hz, 2H), 4.92(s, 1H), 4.52(d, J=5.9Hz, 1H), 4.34-3.68(m, 3H), 3.31(d, J=18.8Hz, 4H), 2.40(s, 1H),2.27-1.88(m,5H),1.83-1.58(m,2H),1.58-1.24(m,8H),1.18(t,J=7.1Hz,2H),1.05(s,4H),0.82 (d,J=6.8Hz,3H),0.61(d,J=6.8Hz,3H); 13 C NMR(100MHz,DMSO)δ217.55,170.79,166.83,161.88,161.51,160.51,141.22,115.85,81.23,73.13,70.83,60.22,57.65,45.43,44.63,43.90,42.04,40.56,40.30,40.14,39.83,39.62 ,39.41,36.82,34.56,30.57,29.38,29.07,27.09,24.93,21.23,16.61,14.89,14.56,11.98; LRMS(ES)calcd[M+H] + for C 27 h 39 N 3 o 5 S 518.2683, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com