1,1,1,3,3,3-hexafluoropropane production method
A production method, the technology of hexafluoropropane, which is applied in the production field of 1,1,1,3,3,3-hexafluoropropane, can solve the problems of long reaction time and low reaction yield, and achieve excellent product yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
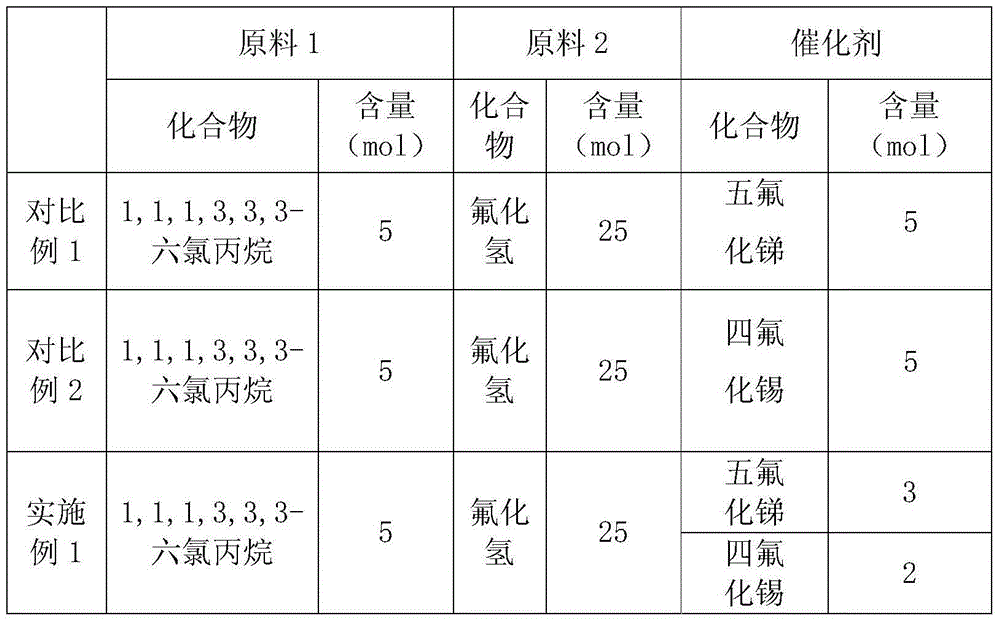
[0016] According to the formula ratio in the following table, the catalyst and 1,1,1,3,3,3-hexachloropropane are added to the reaction kettle at the same time, the oxygen in the kettle is replaced with nitrogen, and then hydrogen fluoride is introduced to carry out the substitution reaction. The reaction temperature is 120°C , normal pressure (101325Pa) environment, the reaction time is 24 to 30 hours, and the crude product after the reaction is distilled under reduced pressure to obtain 1,1,1,3,3,3-hexafluoropropane.
[0017] Table 1 comparative example 1-2 and the raw material proportioning of the embodiment of the present invention 1
[0018]
preparation Embodiment 2
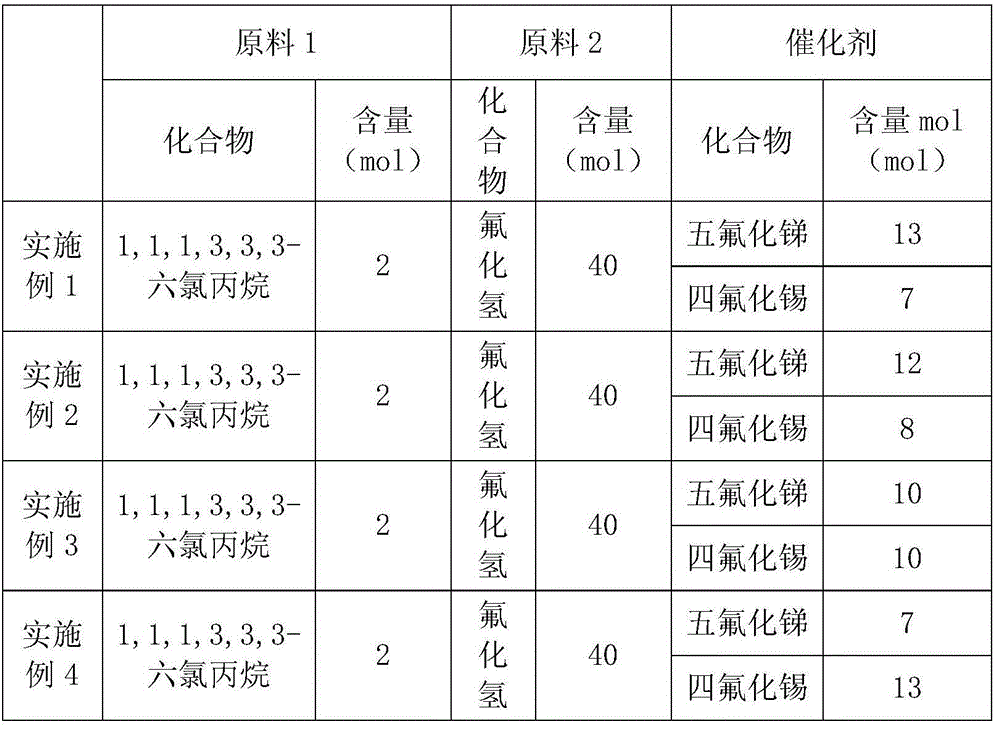
[0025] According to the formula ratio in the following table, the catalyst and 1,1,1,3,3,3-hexachloropropane are added to the reaction kettle at the same time, the oxygen in the kettle is replaced with nitrogen, and then hydrogen fluoride is introduced to carry out the substitution reaction. The reaction temperature is 150°C , the pressure is 20kpa, and the reaction time is 15-18 hours. After the reaction, the crude product is distilled under reduced pressure to obtain 1,1,1,3,3,3-hexafluoropropane.
[0026] Table 3 Raw Material Proportions of Examples 1-7 of the present invention
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com