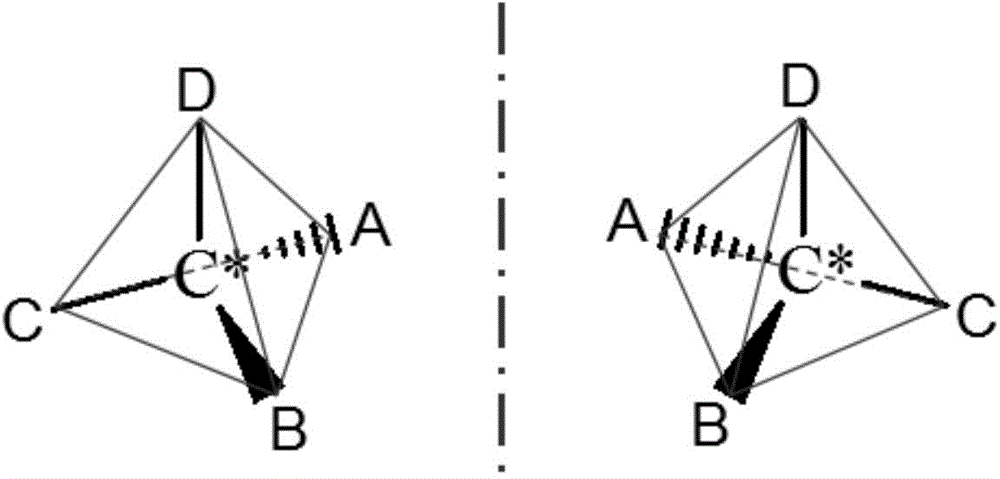
Rapid and nondestructive identification method of optical isomer with biochemical activity
An optical isomer and activity technology, which is applied in the field of rapid and nondestructive identification of biochemically active optical isomers, can solve the problems of rapid and nondestructive identification of biochemically active optical isomers and the identification principle that has not yet been clearly reported, and achieves improved performance. Industrial and agricultural production efficiency, high work efficiency, and the effect of promoting life sciences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Use the following procedure to identify tartaric acid sample A of unknown optical activity:
[0044] (1) Take tartaric acid sample A and β-cyclodextrin, the molar ratio of said tartaric acid sample A and β-cyclodextrin is 1:2.5; fully infiltrate with pure water and mix evenly, air-dried naturally to obtain the adsorption mixture, spare;
[0045] (2) collecting the terahertz-time domain spectral data of the adsorption mixture; the conditions required for collection include:
[0046] Terahertz spectral resolution: 0.0076THz;
[0047] Terahertz spectral frequency: 0.7THz;
[0048] Terahertz spectrum sampling method: attenuated total reflection;
[0049] Terahertz spectral reference: empty optical path;
[0050] Terahertz spectrum accumulation times: 2048 times;
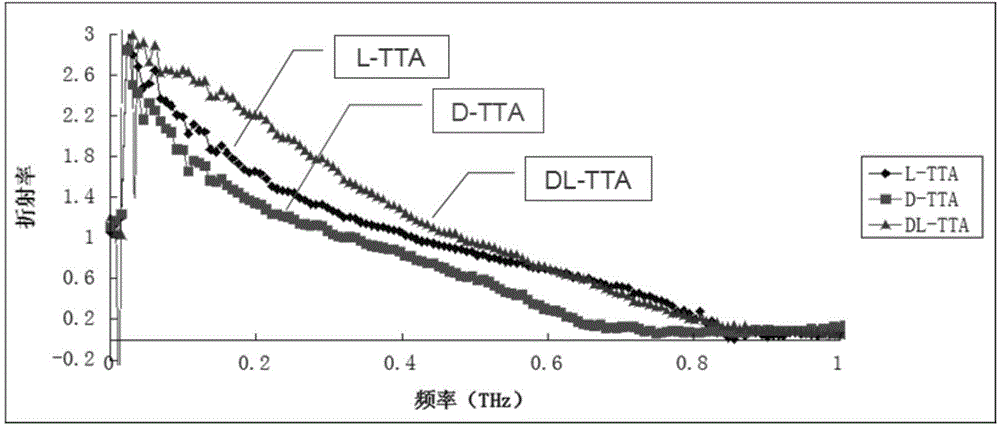
[0051] (3) Through Fourier transform, the terahertz-time domain spectral data is converted into terahertz-refractive index spectrum data; according to the refractive index in the terahertz-refractive index sp...
Embodiment 2
[0054] Compared with Example 1, the only difference is that the molar ratio of tartaric acid sample A to β-cyclodextrin is 1:2, the frequency of the terahertz spectrum is 0.8 THz, and the number of accumulation times of the terahertz spectrum is 2000.
[0055] After testing, the refractive index of the sample was 0.6, and it was determined that the sample was L-tartaric acid with biochemical activity.
Embodiment 3
[0057] Compared with Example 1, the only difference is that the molar ratio of tartaric acid sample A to β-cyclodextrin is 1:3, the terahertz spectral resolution is 0.008THz, the terahertz spectral frequency is 0.6THz, and the number of terahertz spectral accumulations is for 2100 times.
[0058] After testing, the refractive index of the sample was 0.7, and it was determined that the sample was L-tartaric acid with biochemical activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com