Industrial production method applicable to citric acid tofacitinib
A technology of tofacitinib and a production method, which is applied in the industrialized production field of tofacitinib citrate, can solve the problems of increasing production costs and potential safety hazards, no suppliers, and unsatisfactory costs, and achieves high economic value and cost. Social benefits, shortened response time, less harsh production conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
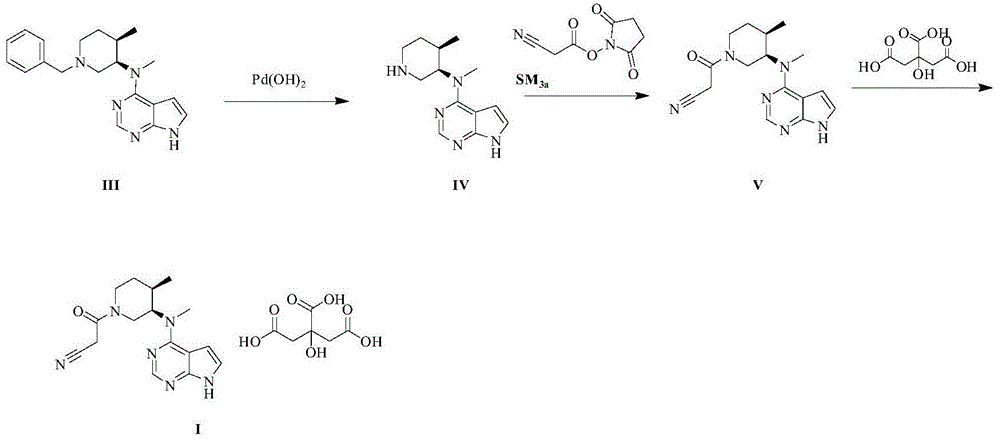
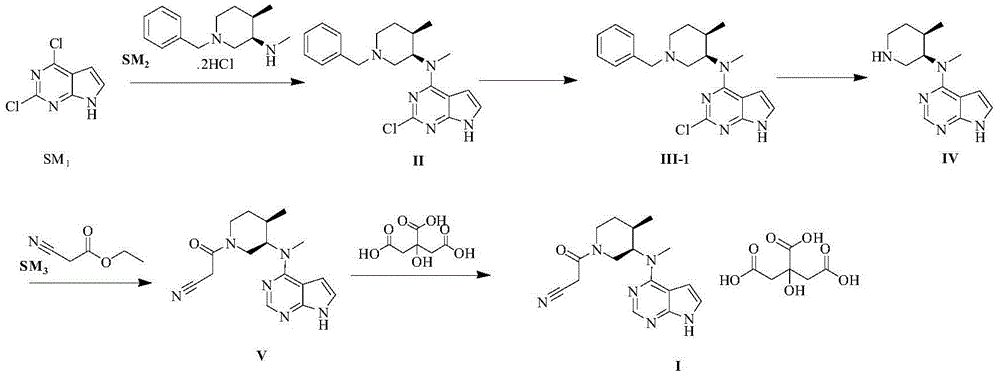
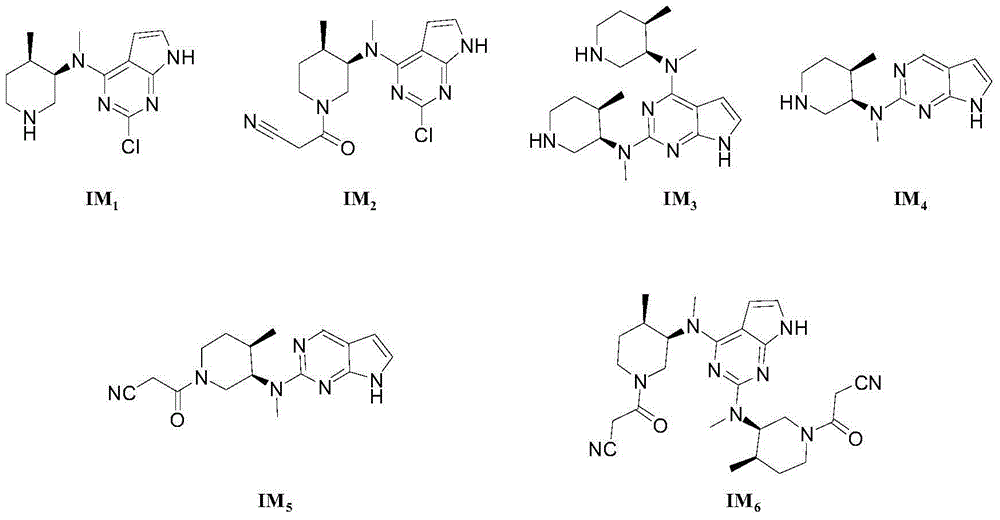
Image
Examples
Embodiment 1
[0036] The specific operation steps of producing tofacitinib citrate are as follows:
[0037] 1. Preparation of formula IIa compound
[0038]
[0039] Drop into 0.7Kg 7H-pyrrole [2,3-D] pyrimidine (formula SM 1a shown compound), 0.6Kg (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride (formula SM 2 shown compound) to the reactor, start stirring, vacuum pump 3kg of methanol successively, put in 0.5kg of powdered potassium carbonate, heat up to 60-65°C, keep stirring for 8 hours, and detect SM by engineering inspection HPLC 1a Remain 1.0%, lower the temperature to 40-45°C, vacuum pump 0.6kg of acetonitrile, heat and stir for 3 hours, centrifuge and dry to obtain a wet product, sprinkle and wash the wet product twice with 1.5kg of water, and dry again to obtain a wet product of 1.68kg, keep Blast drying at 40-50°C for 12 hours gave 0.75kg of off-white solid formula IIa with a yield of 75%, a purity of 97.2%, and SM 1a 0.5% residual, 1.0% moisture residual....
Embodiment 2
[0057] The specific operation steps of producing tofacitinib citrate are as follows:
[0058] 1. Preparation of formula IIa compound
[0059]
[0060] Drop into 0.7Kg 7H-pyrrole [2,3-D] pyrimidine (formula SM 1a shown compound), 0.6Kg (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride (formula SM 2 shown compound) to the reactor, start stirring, vacuum pump 2.8kg methanol successively, put in 0.4kg powdered sodium carbonate, heat up to 60-65°C, keep stirring for 10 hours, and detect SM by engineering inspection HPLC 1a Residual 1.2%, lower the temperature to 40-45°C, vacuum pump 0.56kg of acetonitrile, heat and stir for 3 hours, centrifuge and dry to obtain a wet product, sprinkle and wash the wet product twice with 1.5kg of water, and dry again to obtain a wet product of 1.6kg, keep 40~50 ℃ air-dried for 12 hours, obtain 0.71kg off-white solid formula IIa, yield 72.4%, purity 96.8%, SM 1a 0.8% remains and 1.2% moisture remains.
[0061] 2. Preparation...
Embodiment 3
[0078] The specific operation steps of producing tofacitinib citrate are as follows:
[0079] 1. Preparation of formula IIa compound
[0080]
[0081] Drop into 0.72Kg 7H-pyrrole [2,3-D] pyrimidine (formula SM 1a shown compound), 0.6Kg (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride (formula SM 2 shown compound) to the reaction kettle, vacuum pump 2.8kg isopropanol successively after starting stirring, put in 0.45kg powdered sodium carbonate, heat up to 75-80°C, keep stirring for 12 hours, and detect SM through engineering inspection HPLC 1a Remain 1.3%, lower the temperature to 40-45°C, vacuum pump 0.6kg of acetonitrile, heat and stir for 4 hours, centrifuge and dry to obtain a wet product, sprinkle and wash the wet product twice with 1.8kg of water, and dry again to obtain a wet product of 1.7kg, keep 40~50 DEG C blast drying 14 hours, obtain 0.70kg off-white solid formula IIa, yield 70%, purity 97.3%, SM 1a 1.0% remained and 1.4% moisture remained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com