Adenocarcinoma specificity EpCAM-GM-CSF genetic recombinant fusion protein and preparation method thereof
A technology of fusion protein and genetic recombination, which can be applied in the direction of drug combination, peptide/protein components, chemical instruments and methods, etc., and can solve the problems of poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] 1. Experimental materials
[0148] 1.1 Plasmid:
[0149] Plasmid pORFhGM-CSF was purchased from Invivogen; plasmid pET-30a was purchased from Novagen.
[0150] 1.2 Main reagents:
[0151] Plasmid extraction kits and gel recovery kits were purchased from Shanghai Sangong; Ni SepharoseTM 6Fast Flow was purchased from GE.
[0152] 2. Experimental method:
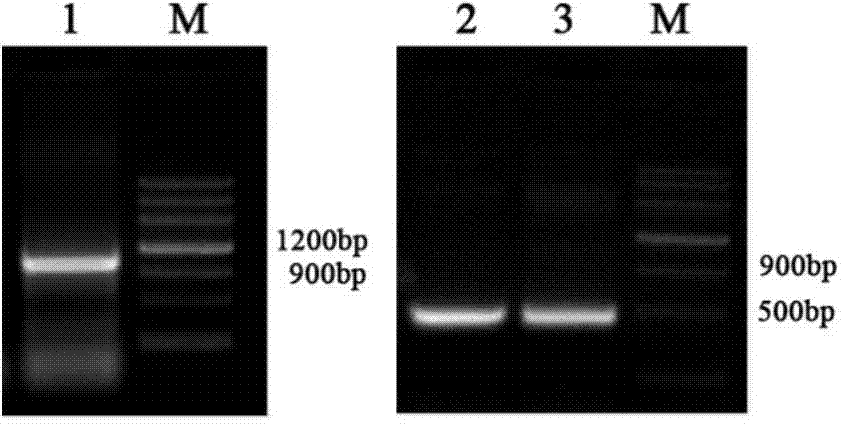
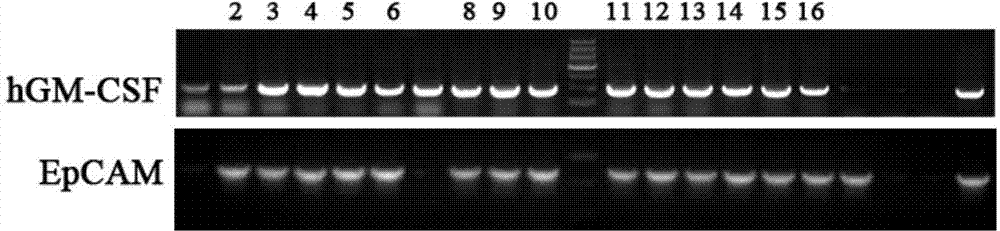
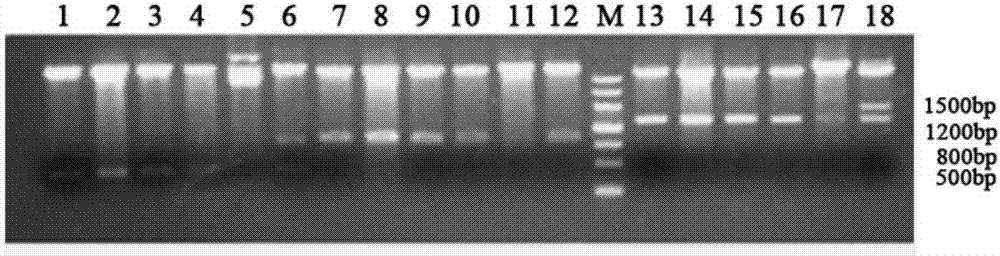
[0153] 2.1 Construction of recombinant EpCAM-GM-CSF fusion protein expression plasmid
[0154] 2.1.1 Design and synthesis of primers:
[0155] According to the restriction site of plasmid pET-30a, the EpCAM gene sequence provided by NCBI, and the gene sequence provided by plasmid pORFhGM-CSF, the upstream and downstream primers of EpCAM and hGM-CSF genes were designed respectively:
[0156] The sequence of the upstream primer of EpCAM is: 5'CATGCCATGGgcATGGCGCCCCCGCAGGT3'; the sequence of the downstream primer is: 5'ACGCGTCGACTGCATTGAGTTCCCTATGCATC 3'; the upstream and downstream primers respectively introduce restr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com