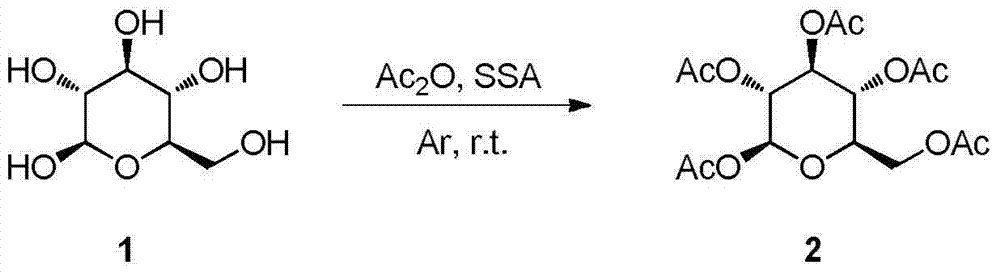
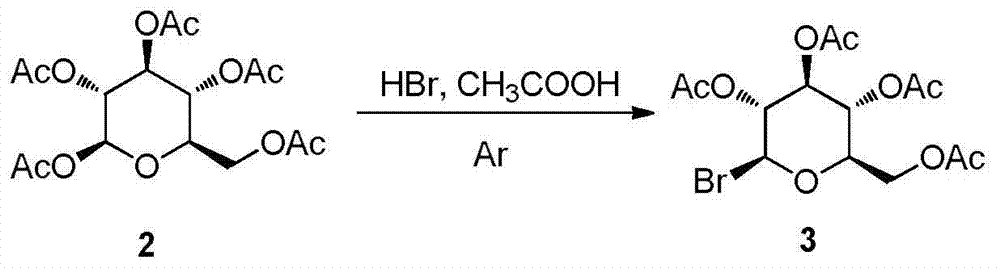
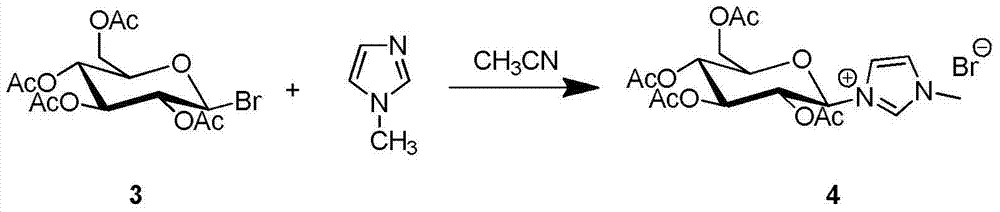
Synthesis and uses of beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate
A technology of glucopyranose and hydrogen sulfide, applied in the field of multi-component reaction chemical synthesis of hydrogen sulfide, which can solve the problems of difficult control of reaction selectivity, increase of reactant types, increase of reactive sites, etc., and achieve high Practical application value, short reaction time, and the effect of reflecting green environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Taking 3-hydroxyl-3-(2-oxo-2-phenylethyl)indol-2-one (10a) as an example:
[0062] Aryl formaldehyde 7a (2mmol), p-methylaniline 8a (2mmol), ethyl acetoacetate 9 (1mmol), catalyst 6 (10mol%) and ethanol (2mL) were sequentially added to a 50mL round-bottomed flask, mixed well, 40 The reaction was complete at ℃ (TLC tracking), cooled the system to room temperature, added 20 mL of distilled water, stirred until a solid precipitated, filtered with suction, and recrystallized (95% EtOH-DMF=1:4) to obtain the product 10a.
[0063] Ethyl 4-(4-tolylamino)-1,2,5,6-tetrahydro-2,6-diphenyl-1-(4-tolylpyridine)-3-carbox ylate(19a)
[0064] 7.28-7.31(m,8H),10.18(s,1H); 13 C NMR (100MHz, CDCl 3 ): δ168.3, 156.4, 144.9, 144.4, 143.1, 135.5, 135.2, 129.5, 129.4, 128.6, 128.2, 127.0, 126.7, 126.5, 126.2, 125.9, 125.0, 112.9, 95.6, 59.6, 08.2, 2 20.1, 14.8; HRMS(ESI) m / z: calc.for C 34 h 34 N 2 o 2 Na[M+Na] + found(expected):525.2513(525.2518)..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com