A supported palladium catalyst adopting nanocarbon as a carrier, preparation thereof and applications of the catalyst
A nano-carbon and catalyst technology, applied in the field of organic catalytic reactions, can solve the problems of loss of Pd nanoparticles and long-term recycling of unfavorable catalysts, and achieve the effects of various product forms, fewer steps, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, comparative example 1
[0046] 1. Sample preparation
[0047] (1) Embodiment 1
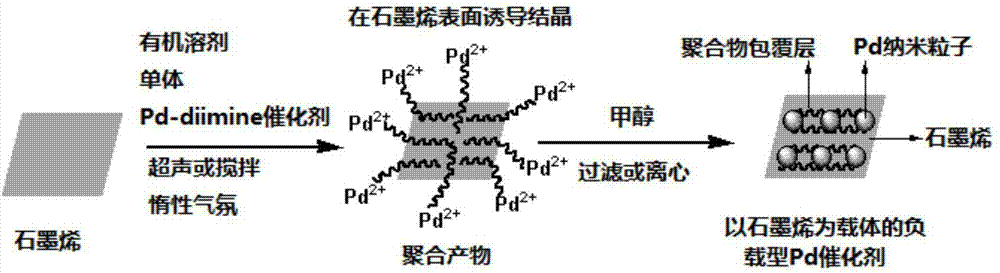
[0048] Step 1: Under the protection of ethylene, inject 100 mL of anhydrous dichloromethane into a 250 mL Schlenk bottle, stir for 30 min to keep the temperature at 35 ° C; then add Pd-diimine catalyst 1 (0.2 g, previously Dissolve in 10 mL of anhydrous grade dichloromethane). After stirring the above solution at a constant polymerization temperature (35° C.) and ethylene pressure (0.1 MPa) to continue the polymerization for 24 h, the resulting product was added to 200 mL of 1% acidified methanol to terminate the polymerization. The resulting polymerized product was first purged with air to remove the solvent at room temperature, and the obtained product was dissolved in 50 mL of tetrahydrofuran (THF). particles; subsequent addition of methanol (100 mL) precipitated the polymer product. In order to further remove a small amount of catalyst ligand contained in the product, ...
Embodiment 2
[0075] Embodiment 2, comparative example 2
[0076] 1. Sample preparation
[0077] (1) Example 2
[0078] Step 1: The preparation process of HBPE is the same as Step 1 in Example 1, and the yield of HBPE is 19.7g.
[0079] Step 2: In a cylindrical glass bottle of 100mL size, add 800mg of natural flake graphite (specification is the same as described in step 2 in Example 1), 160mg HBPE (synthesized by the above step 1) and 80mL chloroform (analytical pure ), sealed and placed in a 250W ultrasonic pool for 48 hours at room temperature. The resulting product was centrifuged at 4000rpm for 45min, and about 70mL of the upper graphene solution (containing excess HBPE) was collected. Further vacuum filter the obtained graphene solution with a polytetrafluoroethylene membrane with a pore size of 0.22 μm, and rinse with fresh chloroform to remove excess HBPE, and add 30 mL of dichloromethane to the solid product after the filtration (analysis Pure), ultrasonic 8h at room temperatur...
Embodiment 3
[0103] Embodiment 3, comparative example 3
[0104] 1. Sample preparation
[0105] (1) Example 3
[0106] Step 1: The HBPE synthesis process is the same as step 1 in Example 1, and the resulting HBPE yield is 20.5 g.
[0107] Step 2: The graphene preparation process is the same as step 2 in Example 1, and a total of 60 mL of graphene solution (with chloroform as solvent, graphene concentration of 0.17 mg / mL) is obtained.
[0108] Step 3: Under the protection of nitrogen, add 42mL of the graphene solution obtained in the above step 2 (the quality of graphene is 7.14mg, and the feeding concentration is 0.12mg / mL) into a 100mL cylindrical glass bottle, and pass ultrasound at room temperature (power 250W, Time 0.5h) obtain graphene initial dispersion liquid; Further add 18mL monomer cyclopentene (13.86g, feeding concentration is 0.03mol / mg graphene) and 450mg Pd-diimine catalyst 2 (feeding concentration is 0.07 mmol / mg graphene), followed by ultrasonic (power 250W) continuous r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com