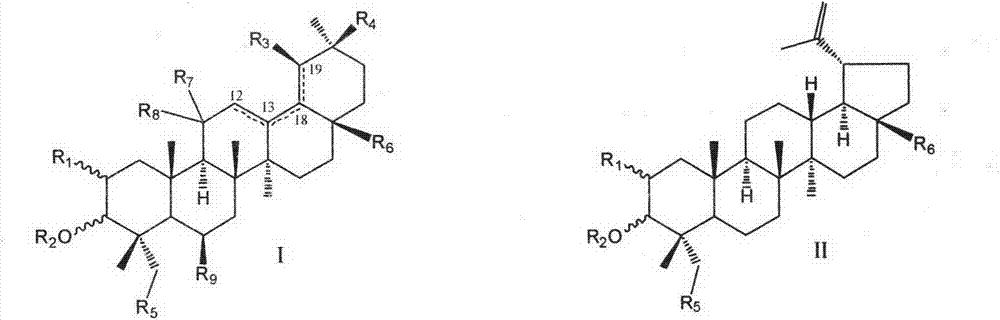
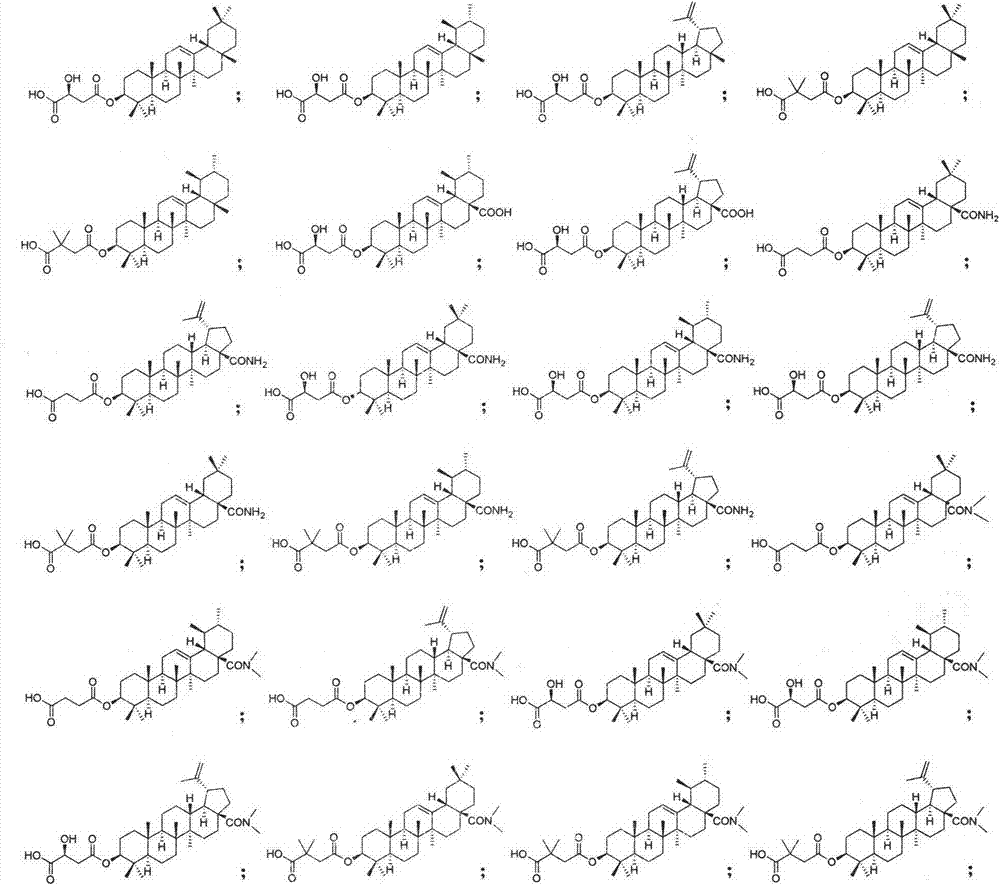
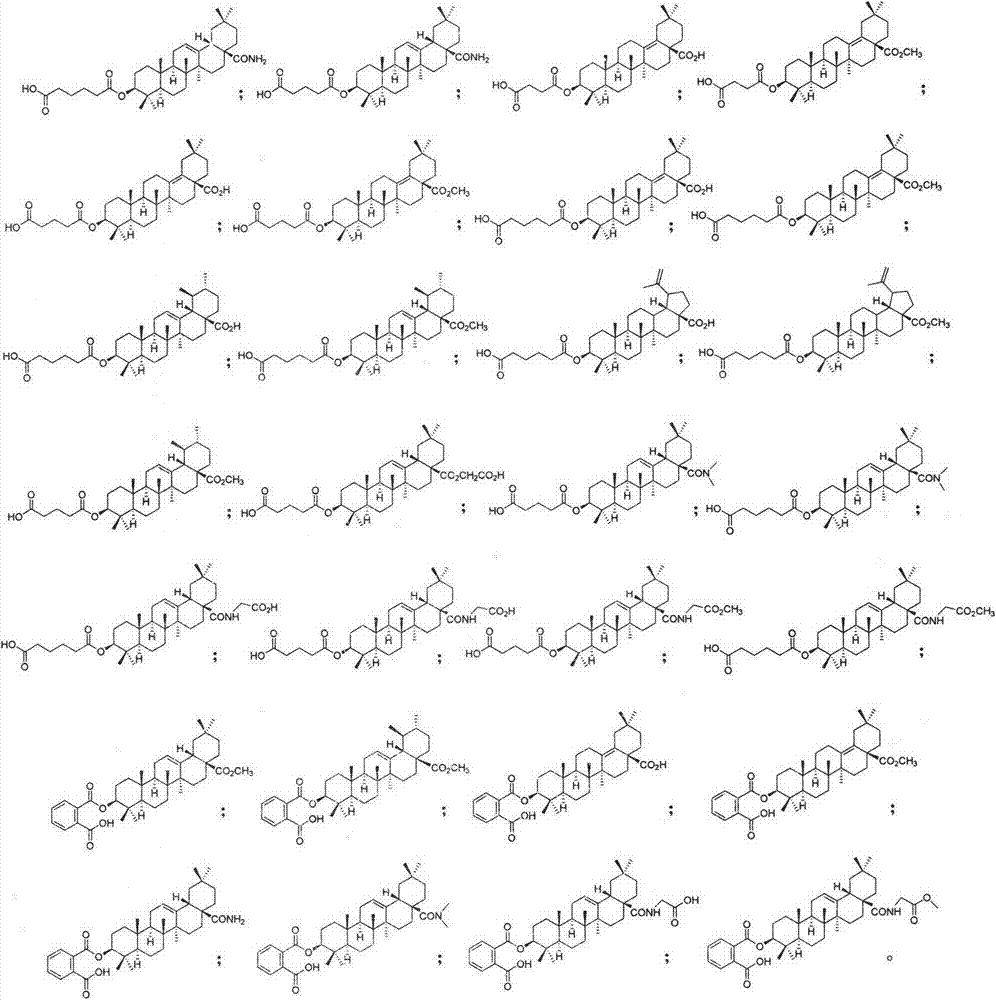
Pentacyclic triterpenoid cholesterol ester transfer protein (CETP) inhibitor, pharmaceutical composition thereof and medical application
A technology of pentacyclic triterpenes and transporters, which is applied in the direction of drug combinations, pharmaceutical formulas, steroids, etc., and can solve the problems of elevated aldosterone levels in the body, inability to be fully absorbed, and inaccessibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 3β-Acetoxy-12-en-28-methyloleanane
[0055] 3β-Hydroxy-12-ene-28-methyloleanane (100 mg, 0.234 mmol) was dissolved in 3 mL of pyridine, acetic anhydride (96 mg, 0.937 mmol) and 4-dimethylaminopyridine (DMAP) (29 mg , 0.234mmol), stirred at room temperature for 24h. The disappearance of raw materials was detected by TLC, the solvent was evaporated to dryness under reduced pressure, 50 mL of water was added, extracted with ethyl acetate (3×10 mL), the organic layers were combined, washed with 1N hydrochloric acid and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure, and a white solid (90 mg, 82%) was obtained by flash column chromatography (petroleum ether: ethyl acetate = 100:1). 1 H NMR (500MHz, CDCl 3 )δ5.18(t, J=3.6Hz, 1H), 4.55-4.46(m, 1H), 2.04(s, 3H), 2.03-0.79(m, 23H), 1.13(s, 3H), 0.97(s , 3H), 0.96(s, 3H), 0.88(m, 6H), 0.87(s, 3H), 0.86(s, 3H), 0.83(s, 3H); ESI MS m / z 491.39[M+Na] + .
Embodiment 2
[0057] 3β-Oleoyloxy-12-en-28-methyloleanane
[0058] 3β-Hydroxy-12-ene-28-methyloleanane (100 mg, 0.234 mmol) was dissolved in 3 mL of dichloromethane, and oleic acid (132 mg, 0.468 mmol), 1-(3-dimethyl Aminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (90mg, 0.468mmol) and DMAP (57mg, 0.468mmol), heated to 50°C and stirred for 6h. TLC detected the disappearance of the starting material, added 50 mL of water, extracted with dichloromethane (3×10 mL), combined the organic layers, washed with 1N NaOH aqueous solution and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure, and a white solid (145 mg, 90%) was obtained by flash column chromatography (petroleum ether: ethyl acetate = 400:1). 1 H NMR (300MHz, CDCl 3 )δ5.34(s, 2H), 5.18(s, 1H), 4.56-4.45(m, 1H), 2.29(t, J=7.3Hz, 2H), 2.02-0.66(m, 52H), 1.13(s , 3H), 0.97(s, 6H), 0.87(s, 12H), 0.83(s, 3H); ESI MS m / z 691.16[M+Na] + .
Embodiment 3
[0060] 3β-Succinic acid monoacyloxy-12-en-28-methyloleanane
[0061] 3β-Hydroxy-12-ene-28-methyloleanane (100mg, 0.234mmol) was dissolved in 3mL of pyridine, succinic anhydride (94mg, 0.937mmol) and DMAP (29mg, 0.234mmol) were added, heated to 110°C, stirring for 12h. The disappearance of raw materials was detected by TLC, the solvent was evaporated to dryness under reduced pressure, 50 mL of water was added, extracted with ethyl acetate (3×10 mL), the organic layers were combined, washed with 1N hydrochloric acid and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated to dryness under reduced pressure, and a white solid (92 mg, 75%) was obtained by flash column chromatography (petroleum ether: ethyl acetate = 5:1). 1 H NMR (300MHz, DMSO-d 6 )δ5.18(s, 1H), 4.52(t, J=7.8Hz, 1H), 2.78-2.53(m, 4H), 2.00-0.70(m, 23H), 1.13(s, 3H), 0.96(s , 3H), 0.94(s, 3H), 0.92(s, 3H), 0.87(s, 6H), 0.82(s, 3H), 0.75(s, 3H); ESI MS m / z 549.78[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com