Cationic liposomal drug delivery system for specific targeting of human cd14+ monocytes in whole blood
A cationic lipid and liposome technology, applied in liposome delivery, cardiovascular system diseases, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Liposome preparation
[0153] Monolamellar fully hydrated liposomes are composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate Glycerol (POPG), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly Ethylene glycol)-2000] (DOPE-PEG2000) mixture. As a fluorescent label to determine the presence of liposomes in biological systems, 0.5% 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-rhodamine (DOPE-RhB) was combined with lipid as tracer mix. Summarize the molar ratio of each lipid in the liposomes in figure 1 In A. All lipids were obtained from Avanti Polar lipids. Briefly, weighed appropriate amounts of POPC, POPG, DOTAP and DOPE-PEG2000 were dissolved in chloroform. by Moderate N 2 The solvent was removed by airflow and the lipid film was dried overnight at low pressure to remove traces of solvent. Multilamellar liposomes were prepared by dispers...
Embodiment 2
[0155] Characterization of composition-dependent liposome size and surface charge
[0156] Preparations such as figure 1 Liposomes prepared are summarized in A. We designed liposomes with 0-50% net positive charge ( figure 1 Formulations 6-12 in A) were compared with negatively or nearly neutrally charged control liposomes ( figure 1 Formulations 1-5 in A). Liposomes were prepared as described in Example 1 and prepared in MilliQ water from 300 mM glucose, 10 mM HEPES, 1 mM CaCl by dynamic light scattering using a ZetaPALS zeta potential analyzer from Brookhaven Instruments. 2 Their size in nanometers (nm) was determined in a pH 7.4 buffer solution. Liposomes show a diameter of 110 ± 20nm ( figure 1 B). The surface charge (zeta potential) of the liposomes was measured in mV and it was shown that the surface charge is dependent on the lipid composition ( figure 1 C). The addition of negatively charged POPG (10 mol%) to liposomes revealed a negative surface charge of -13m...
Embodiment 3
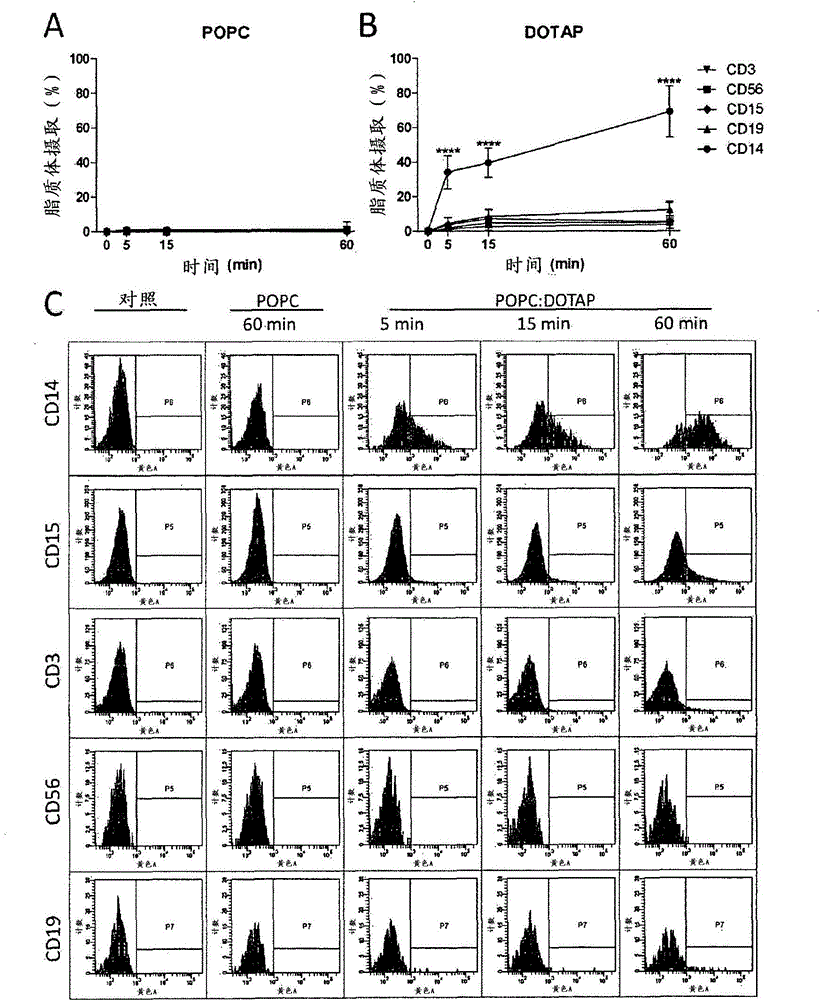
[0158] Liposome composition-dependent targeting of liposomes to monocytes
[0159] Cellular uptake of improved POPC liposome formulations was determined based on the fluorescence of RhB incorporated into the liposomal membrane. The total amount of cell-associated liposomes (expressed as 'uptake' and including cell membrane-associated liposomes and liposomes that have been internalized) was assessed using excitation at 532 nm and emission at 564-606 nm. . Liposome uptake in 5 different cell populations in whole blood was analyzed. The following markers were used to differentiate the different populations: CD14 (monocyte marker), CD15 (granulocyte marker), CD3 (T-lymphocyte marker), CD19 (B-lymphocyte marker) and CD56 (natural killer cell marker). Whole blood was obtained from healthy volunteers by standard methods using BD vacuum blood collection tubes containing ethylenediaminetetraacetic acid (EDTA, 366450). Briefly, 10 μl of liposome formulation ( figure 1 Formulations 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com