biomimetic electrochemical cell
An electrochemical and electrode technology, applied in the direction of biochemical fuel cells, electrochemical generators, fuel cells, etc., can solve the problems of relatively few studies on the forced transfer process of electrochemical cells, reduce the probability of side reactions, and enhance diffusion , the effect of preventing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1. The electrochemical reaction process that does not consume gas and liquid reactant
[0058] The design of a biomimetic electrochemical cell for such a process is illustrated using the electrolytic refining of copper as an example. First, the electrolytic copper reacts as follows:
[0059] Blister copper anode: Cu=Cu 2+ +2e
[0060] Refined copper cathode: Cu 2+ +2e=Cu
[0061] Total reaction: Cu (粗) =Cu (纯)
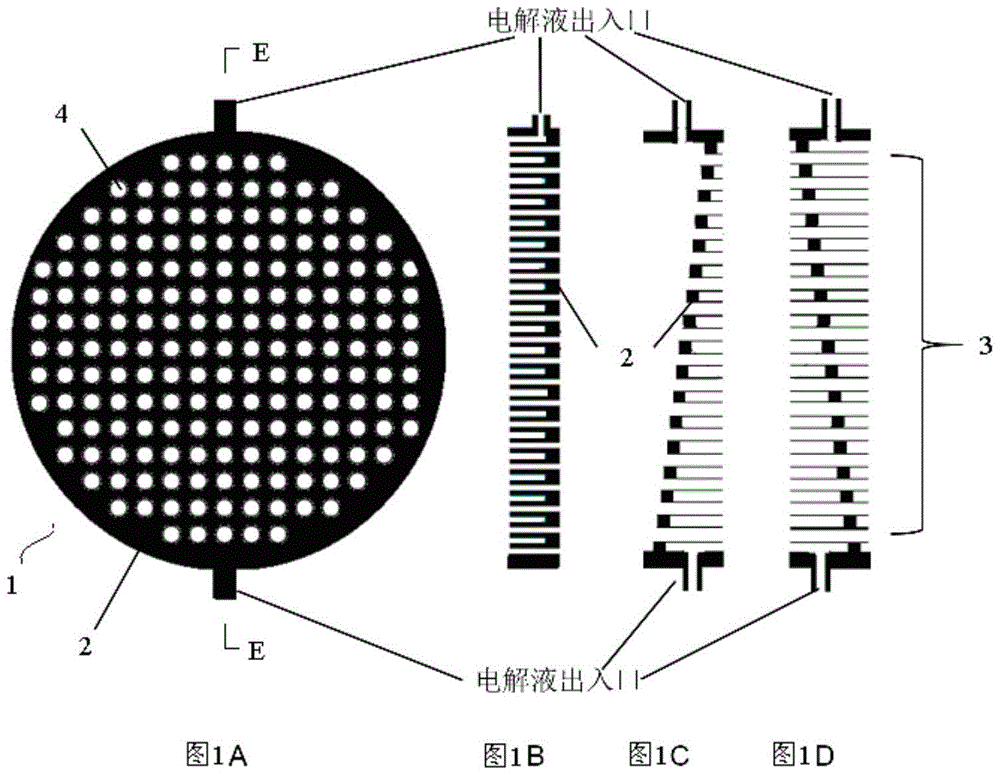
[0062] Its electrolyte is CuSO 4 and H 2 SO 4 of the mixture. Therefore, the electrolyte enters from one end of the cathode, where copper ions obtain electrons and deposit on the refined copper cathode, and the excess SO 4 2- Under the control of the deflector, it is entrained by the electrolyte and flows from the pipeline array to the anode of the thick copper plate without back mixing, where SO 4 2- Cu produced by anodic oxidation 2+ Combine and leave the anode area. Because the anode dissolution process of blister copper will cause ...
Embodiment 2
[0064] Example 2. Electrochemical Reaction Process Consuming Gas or Liquid Reactants on Only One Electrode
[0065] With sacrificial zinc anode CO 2 The electrochemical reduction reaction is illustrated as an example. The reaction consumes the gaseous reactant CO 2 The electrochemical reaction:
[0066] CO 2 Cathode: 2CO 2 +2e=C 2 o 4 2-
[0067] Zinc anode: Zn+C 2 o 4 2- =ZnC 2 o 4 +2e
[0068] Total reaction: Zn+2CO 2 =ZnC 2 o 4
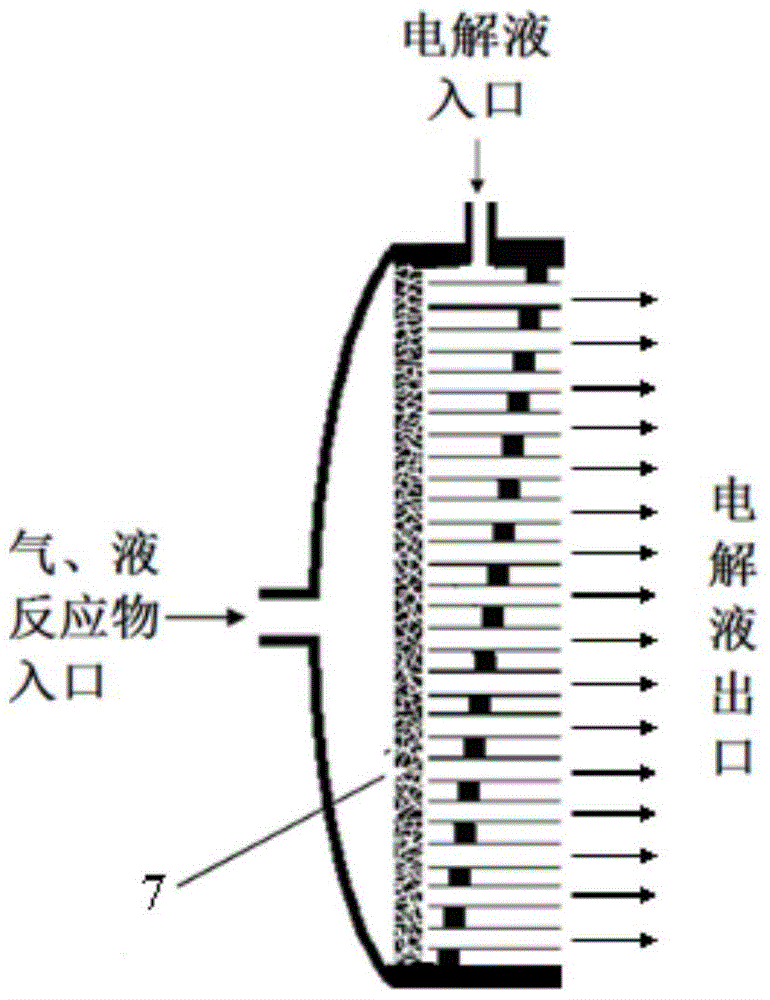
[0069] Its electrolyte is anhydrous tetrabutylammonium perchlorate in acetonitrile. Therefore, CO 2 It enters from one side of the porous cathode, and the electrolyte enters from the other side of the porous cathode, and the C produced by the reaction 2 o 4 2- Brought into the anode area through the pipeline array; then, the C containing C 2 o 4 2- The electrolyte reacts directly with the metal zinc, and then the ZnC produced 2 o 4 into the solid-liquid separator. ZnC 2 o 4 The clean electrolyte obtained after separati...
Embodiment 3
[0071] Example 3. Electrochemical reaction process for gas generation on one or both electrodes
[0072] Take the charging process of a rechargeable zinc-air battery as an example. The process reacts as follows:
[0073] Anode: 2OH - =1 / 2O 2 +H 2 O+2e
[0074] Cathode: ZnO+H 2O+2e=Zn+2OH -
[0075] Total reaction: ZnO=Zn+1 / 2O 2
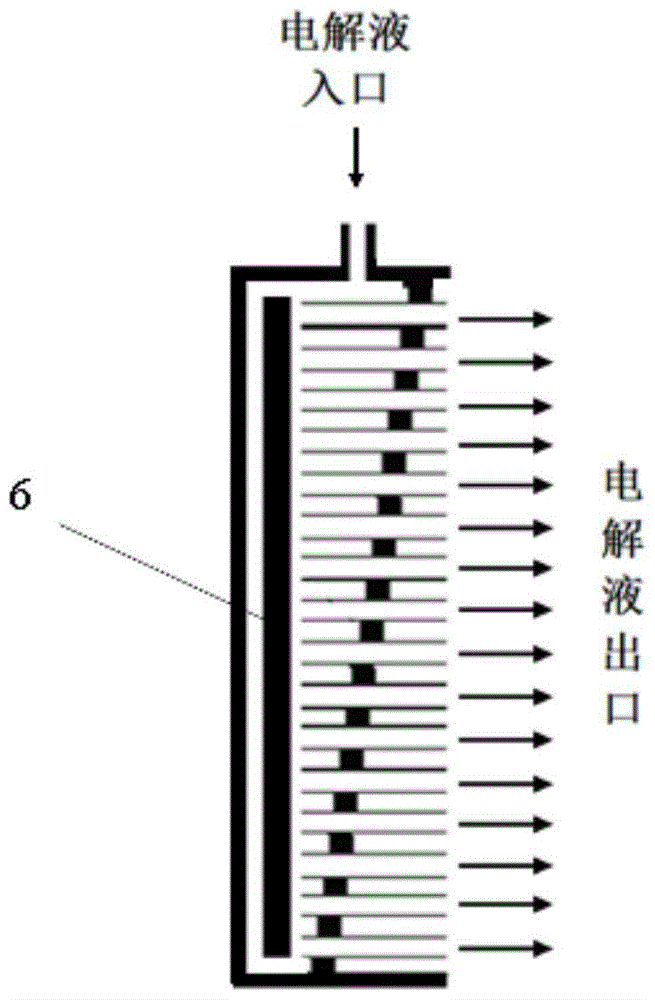
[0076] Its electrolyte is KOH solution. Therefore, the electrolyte enters from one side of the porous anode, and then carries the oxygen formed to the gas-liquid separator, where the oxygen escapes from the reaction system. After that, the electrolyte enters the cathode area, contacts and reacts with zinc oxide to deposit zinc on the electrode, and then the generated OH - out of the reaction system. If there is solid ZnO taken away by the electrolyte, solid-liquid separation can be done afterwards, otherwise the electrolyte can be directly pumped into the anode for recycling ( Figure 6 ).
[0077] For the electrochemical reaction in whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com