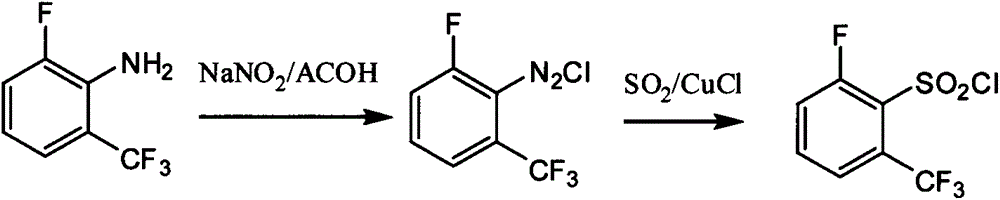
Preparation method of 6-substituted-2-trifluoromethylbenzenesulfonyl chloride
A technology of trifluoromethylbenzenesulfonyl chloride and chlorine substitution, which is applied in the preparation of sulfonic acid and organic chemistry, can solve the problems of complex reaction control conditions, low product yield, and difficult separation, and achieve simple process control conditions and high product quality. The effect of high yield and easy price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of 2,3-difluorobenzotrifluoride
[0051]
[0052] Put 215g (1.00mol) of the compound of formula (II) 2,3-dichlorobenzotrifluoride, 140g (2.41mol) of anhydrous potassium fluoride, 2000mL of sulfolane, and 10g of tetraphenylphosphine bromide into the autoclave in batches, Under stirring, heat up to 250-300° C. and keep warm for 20 hours. Cool and filter to remove the generated potassium chloride and excess potassium fluoride. The filtrate was rectified, and the fraction from 112°C to 119°C was collected to obtain the compound 2,3-difluorobenzotrifluoride of formula (III); the residue continued to be rectified to collect the fraction at 145°C-151°C to obtain the by-product 2-fluoro-3 -Chlorotrifluorotoluene; the by-product can be recycled and applied. After applying mechanically, the average yield of continuous preparation formula (III) compound 2,3-difluorobenzotrifluoride is: 87.6%, HNMR (CH 3 OD, 300 MHz): δ 7.35-7-422 (m, 2H), 7.17-7...
Embodiment 2
[0053] Embodiment 2: Preparation of 6-fluoro-2-trifluoromethyl phenethyl sulfide
[0054]
[0055] Add 182g (1.00mol) of compound 2,3-difluorobenzotrifluoride of formula (III), 500mL of solvent N,N-dimethylformamide, and 200g (1.45mol) of acid-binding agent potassium carbonate into the reaction flask respectively, and place on ice Under bath cooling, control -5°C-5°C, add 65 g (1.05 mol) of ethanethiol dropwise, and drop it in about 3 hours. After the drop, the temperature was raised to 50°C to continue the reaction for 3 hours. The reaction was followed by GC, and the reaction was terminated when the content of the raw material 2,3-difluorobenzotrifluoride was less than 3%. Remove the generated potassium fluoride and excess potassium carbonate by filtration, rectify the filtrate under reduced pressure, and collect the fraction at 115-125°C (50-80mmHg) to obtain the compound 6-fluoro-2-trifluoromethylbenzene of formula (IV) Thioether 175g, yield: 78.13%, content: 96.3%; H...
Embodiment 3~10
[0056] Embodiment 3~10: the preparation of formula (IV) compound 6-fluoro-2-trifluoromethyl phenyl alkyl sulfide
[0057]
[0058] Synthetic method is with embodiment 2, and the ethanethiol in embodiment 2 is changed into corresponding R in table 1 1 The hydrocarbon thiol (M is hydrogen) or its alkali metal salt (M is a metal ion) is reacted in a corresponding solvent, and the experimental data are shown in Table 1.
[0059] Synthetic method and data of table 1 formula (IV) compound
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com