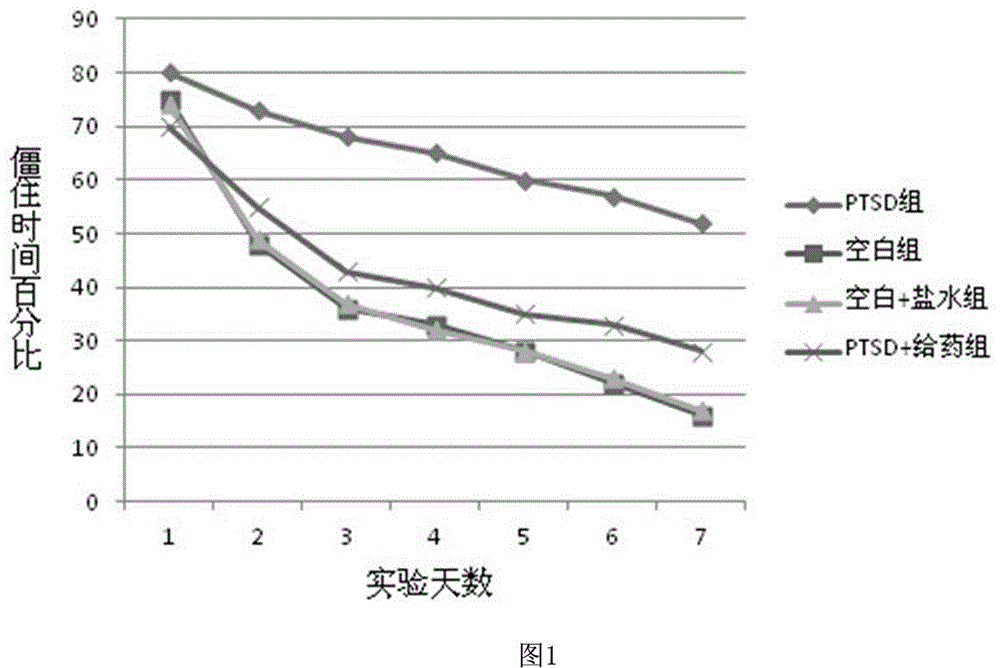
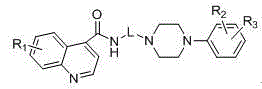
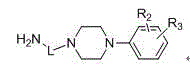Novel quinoline-4-carboxamide derivative, preparation method thereof, pharmaceutical composition containing novel quinoline-4-carboxamide derivative, and medical application of novel quinoline-4-carboxamide derivative
A formamide and compound technology, which is applied in the field of novel quinoline-4-carboxamide derivatives and their preparation, can solve problems such as compatibility stability, safety effects of metabolic drugs in vivo, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Preparation of compound (1): N-{3-[4-(5-chloro-2-methylphenyl)piperazinyl]propyl}-quinoline-4-carboxamide
[0068] (1) Quinoline-2,4-dicarboxylic acid: Weigh 1.0g (6.75mmol) of isatin and 0.7g (8.10mmol) of pyruvic acid and dissolve them in 25ml of 20% NaOH solution, set the microwave power to 110W, React for 10 minutes, stop the reaction, cool to room temperature, acidify with 1M hydrochloric acid solution to a pH value of about 2, a yellow solid precipitates, filter the solid with suction, wash thoroughly with water, and dry at 60°C for 12 hours to obtain the crude product of quinoline-2,4-dicarboxylic acid , dissolved in hot water, decolorized with activated carbon, filtered, and recrystallized to obtain 1.30 g of pure quinoline-2,4-dicarboxylic acid, with a yield of 89.2%. Mp: 235-240°C, 1 H NMR (DMSO- d 6 ): δ =8.81 (d, J = 7.6 Hz, 1H), 8.48 (s, 1H), 8.26 (d, J = 7.8 Hz, 1H), 7.95 (m, 1H), 7.86(m, 1H).
[0069] (2) Quinoline-4-carboxylic acid: Weigh 1.1g (5...
Embodiment 2
[0075] Preparation of compound (2): N-{3-[4-(5-chloro-2-methylphenyl)piperazinyl]propyl}-5-fluoroquinoline-4-carboxamide
[0076] (1) 5-Fluoro-quinoline-4-carboxylic acid: According to the method of Example 1, 4-fluoroisatin was prepared by microwave-catalyzed Pfitzinger reaction with pyruvic acid under alkaline conditions. Yield 83.4%, Mp: 265-269°C. 1 H NMR (DMSO- d 6 ): δ = 9.12 (d, J = 4.5 Hz, 1H), 8.73 (d, J = 8.7 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 8.0 (d, J = 4.5 Hz, 1H), 7.89-7.79 (m, 1H).
[0077] (2) N-{3-[4-(5-chloro-2-methylphenyl)piperazinyl]propyl}-5-fluoroquinoline-4-carboxamide: According to the method of Example 1, take 5-Fluoroquinoline-4-carboxylic acid to prepare 5-fluoroquinoline-4-formyl chloride, and then react with 3-[4-(5-chloro-2-methylphenyl)piperazinyl]propylamine, after deacidification prepared by acylation in the presence of sodium carbonate. Yield: 80.1%, Mp: 263-265°C. 1 H NMR (CDCl 3 ): δ = 1.64(m, 2H), 2.23(s, 3H), 3.10-3.22(m, 4...
Embodiment 3
[0079] Preparation of compound (3): N-{3-[4-(5-chloro-2-methylphenyl)piperazinyl]propyl}-5-trifluoromethylquinoline-4-carboxamide
[0080] According to the method of Example 1, take 5-trifluoromethyl-quinoline-4-formic acid to prepare 5-trifluoromethyl-quinoline-4-formyl chloride, and then combine with 3-[4-(5-chloro-2 -Methylphenyl) piperazinyl] propylamine reaction, prepared by acylation in the presence of acid-removing agent sodium carbonate. Yield: 82.7%. 1 H NMR (DMSO- d 6 ): δ = 1.47(br-s, 2H), 1.52(br-s, 2H), 2.36(s, 3H), 3.38(br-m, 4H), 3.29(q, J = 5.00 Hz, 2H), 3.35(d, J = 8.05Hz,2H), 7.15(m, 2), 7.26(d, J = 6.80 Hz,1H), 7.24(d, J = 8.10 Hz, 1H), 7.58(d, J = 1.92 Hz, 1H), 7.73(dd, J 1 = 8.22Hz, J 2 = 1.62Hz, 1H), 8.43(br-s, 1H,NH), 9.85(br-s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com