Application of recombinant human fibroblast growth factor 21 in prevention and treatment of atherosclerosis and related diseases
A technology of human fibroblasts and atherosclerosis, which is applied in the application field of proteins in the diagnosis or treatment of diseases related to coronary heart disease. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
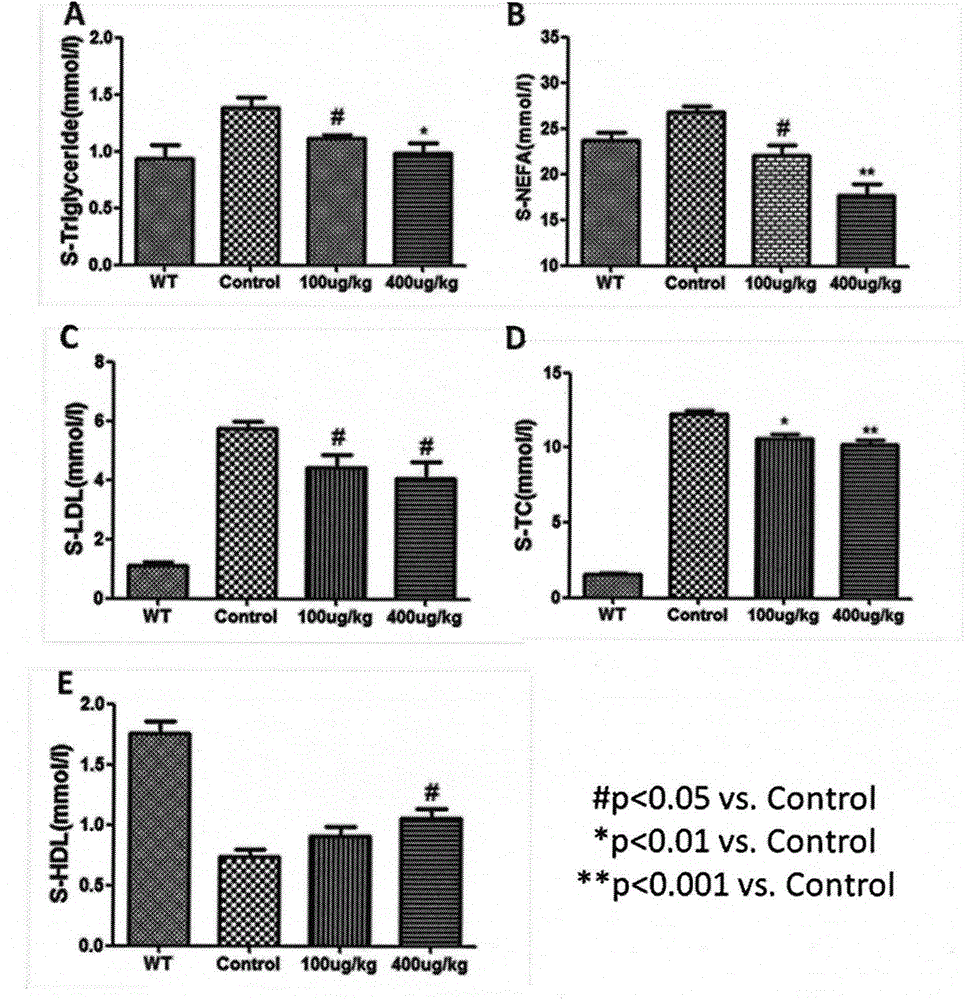
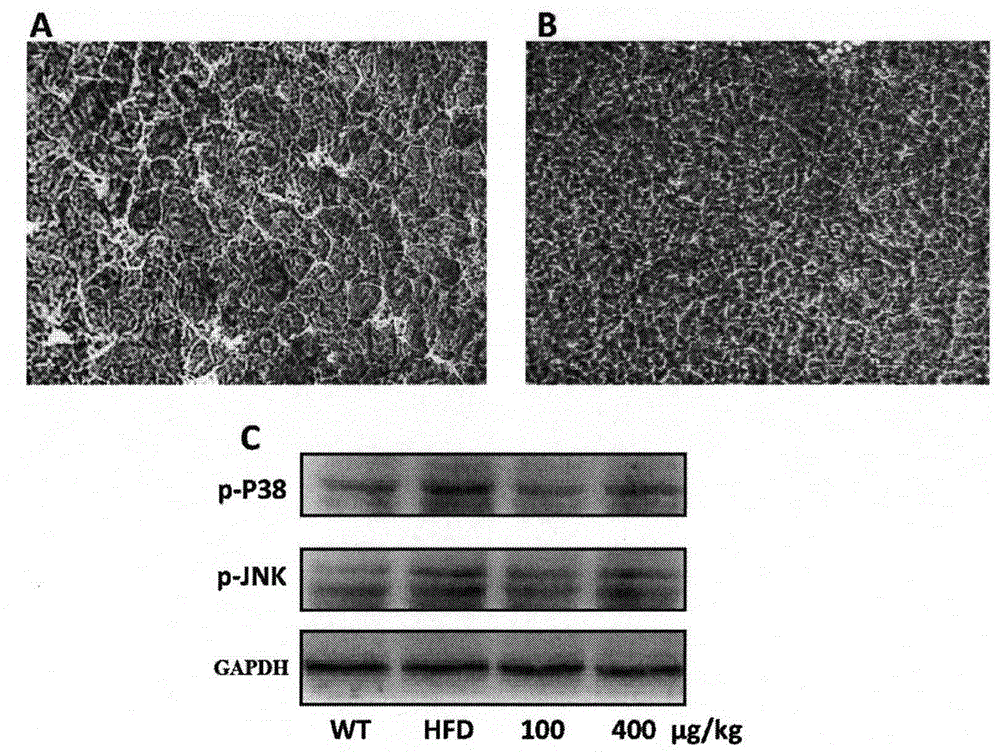
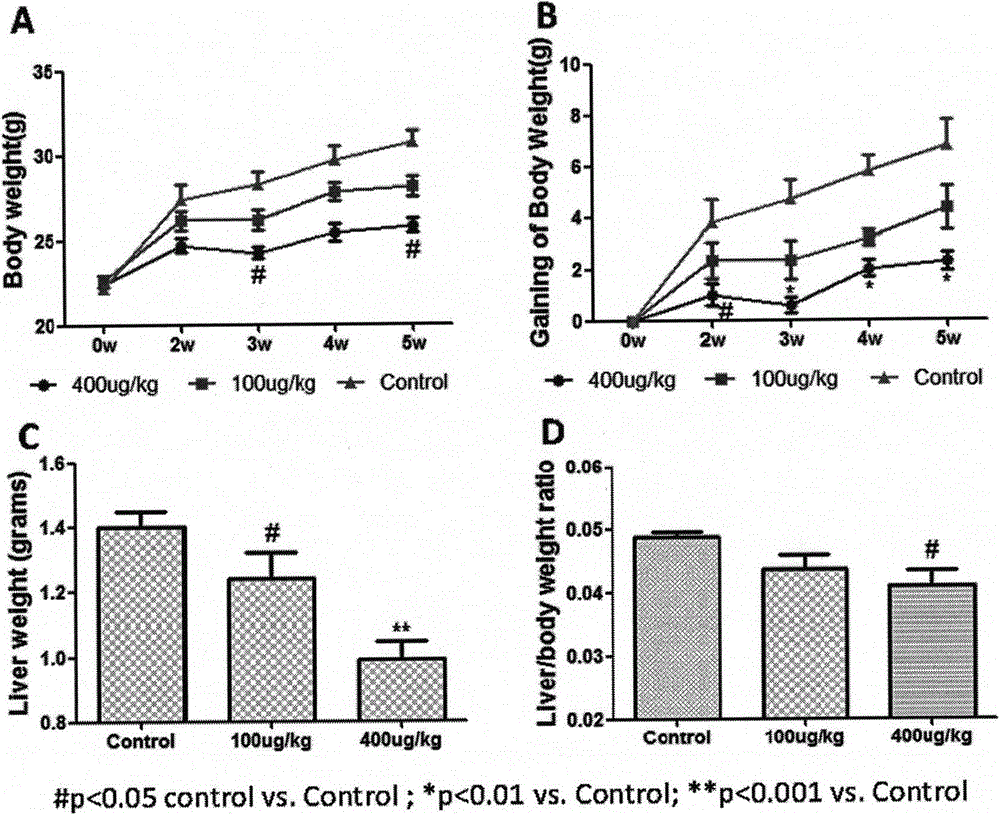
[0039] Example 1 Recombinant human fibroblast factor 21 prevents atherosclerosis
[0040] 1. Animal grouping and drug treatment arrangement
[0041] Thirty 8-week-old ApoE KO mice were divided into three groups, A / B / C, with 10 mice in each group. In addition, 10 C57BL / 6j mice with the same genetic background were used as the normal control group (Group D) , Groups A / B / C were given high-fat diet, and groups A / B were subcutaneously injected with recombinant FGF21 protein at doses of 100 and 400 μg / kg respectively, and groups C / D were injected with normal saline subcutaneously as a control After two weeks, the mice in the four groups A / B / C / D were uniformly fed with normal feed. During the administration process, the body weight of the mice was detected every 2 weeks to observe the body weight change.
[0042] 2. Animal sacrifice and tissue sample collection
[0043] After 6 weeks of administration, the mice were sacrificed, the mice were fasted overnight, anesthetized with ethe...
Embodiment 2
[0134] Clinical manifestations of embodiment 2FGF21 in patients with coronary heart disease
[0135] 1. CHD clinical case collection and clinical analysis methods
[0136] (1) Clinical diagnostic criteria
[0137] According to the "nomenclature and diagnostic criteria for ischemic heart disease" issued by the World Health Organization (WHO) in 1999 and the current clinical diagnostic criteria for CHD in my country, patients with coronary heart disease with angina pectoris or myocardial infarction were listed as the research objects. The specific diagnostic criteria are as follows: ①The clinical diagnosis of myocardial infarction is based on the patient’s ECG test results, elevated serum enzymes in serological tests, and clinical symptoms such as chest discomfort. ②Angina pectoris is based on the following clinical manifestations and detection indicators as the diagnostic criteria, that is, the main manifestation is frequent emotional excitement or chest discomfort for 15 minu...
Embodiment 3
[0157] Embodiment 3 The expression of FGF21 in the mouse model of atherosclerosis
[0158] 1. Grouping of animals
[0159] 20 8-week-old apoE -/- Male mice were randomly divided into two groups A / B, 10 in each group. Group A was fed with high-fat diet, and group B was fed with normal diet. At the same time, 10 normal C57 / BL6j mice were taken as the experimental control group (group C), and fed with normal diet.
[0160] 2. Collection of mouse blood samples
[0161] Before the start of the animal experiment, the above two groups A / B were fasted overnight, blood was collected from the fundus vein, the serum was separated, stored in a -80°C refrigerator, and used as the serum FGF21 test specimen of 8-week-old mice; then at 12 and 24 weeks respectively Blood samples were collected at age, and the changes of serum FGF21 circulating levels were observed.
[0162] 3. Animal sacrifice and tissue sample collection
[0163] When the animals in each group reached the age of 24 weeks...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com