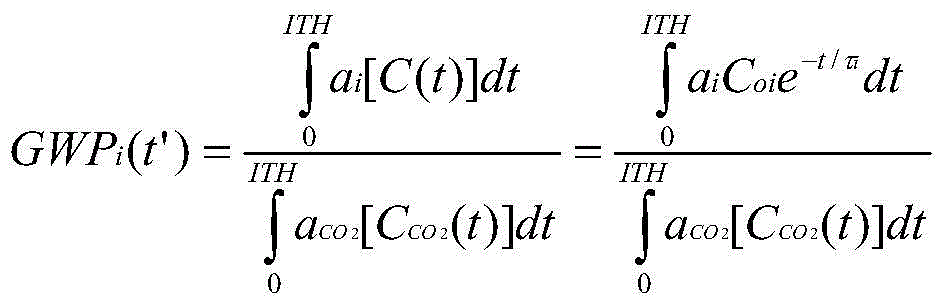Fluorinated nitriles as dielectric gases
A dielectric and gas technology, applied in the direction of inorganic gas, organic gas insulator, organic liquid insulator, etc., can solve the problems of reduced environmental impact, high GWP, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0058] Preparation Example 1: Heptafluoroisobutyramide (CF 3 ) 2 CFCONH 2 Synthesis .
[0059] 100 grams (0.44 mol) of methyl heptafluoroisobutyrate (which was neutralized by essentially US Patent No. 2,713,593 (Brice et al.) R.E. Banks, Preparation, Properties and Industrial Applications of Organofluorine Compounds, pages 19-43 , Halsted Press, New York (1982) (R.E. Banks, Preparation, properties and industrial applications of organofluorine compounds, pp. 19-43, Halsted Press, New York, 1982) Simons of the type described Electrochemical fluorination of isobutyric anhydride in an ECF unit followed by distillation and treatment of the resulting acid fluoride with methanol) and 100 ml of methanol were added to a 250 ml round bottom flask with a magnetic stirrer, thermocouple and dry ice condenser. 12.5 grams (0.74 mol) of ammonia was slowly bubbled into the liquid layer in the flask. The temperature was kept below 40°C. After the ammonia addition was complete,...
preparation example 2
[0060] Preparation Example 2: Synthesis of Heptafluoroisobutyronitrile (CF 3 ) 2 CFCN
[0061] 69.4 grams (0.326mol) of (CF 3 ) 2 CFCONH 2 Dissolve in 154 g of dimethylformamide. The amide / solvent mixture was added to a 500 ml 3 necked round bottom flask equipped with a top vent with manual shut off valve, thermocouple, magnetic stirrer, dry ice condenser, dry ice condenser and addition funnel. The contents of the flask were cooled to -10°C and 51 grams (0.65 mol) of pyridine was added slowly using an addition funnel. 70 grams (0.33 mol) of trifluoroacetic anhydride was slowly added to the flask using an addition funnel. The temperature was maintained at about 0°C throughout the addition. Open the shut-off valve and take material from the top while warming the autoclave to 15°C. 47.6 g of (CF 3 ) 2 CFCN, the yield is 74.9%. Structure confirmed by GC / MS, H-1 and F-19 NMR.
example 2
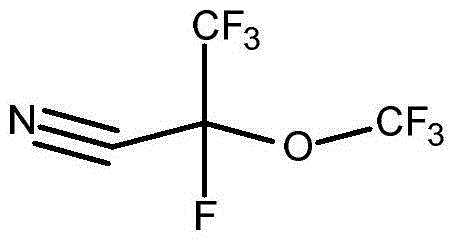
[0063] Preparation of 2,3,3,3-tetrafluoro-2-(trifluoromethoxy)propionitrile
[0064]
[0065] Methyl 2,3,3,3-tetrafluoro-2-methoxypropionate is commercially available (Synquest Laboratories) or produced by adding hexafluoropropylene oxide to methanol to produce Esters are prepared by known methods. Using essentially U.S. Patent No. 2,713,593 (Brice et al.) and R.E. Banks, Preparation, Properties and Industrial Applications of Organofluorine Compounds, pages 19-43, Halsted Press, New York (1982) (R.E. Banks, Preparation of Organofluorine Compounds) , Properties and Industrial Applications, pp. 19-43, Halsted Press, New York, 1982) in a Simons ECF unit of the type described by electrochemical fluorination of 2,3,3,3-tetra Methyl fluoro-2-methoxypropionate is converted to 2,3,3,3-tetrafluoro-2-(trifluoromethoxy)propionyl fluoride.
[0066] 2,3,3,3-Tetrafluoro-2-(trifluoromethoxy)propionyl fluoride (195 g) was added to a 500 mL round bottom flask. The flask was kept cool u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com