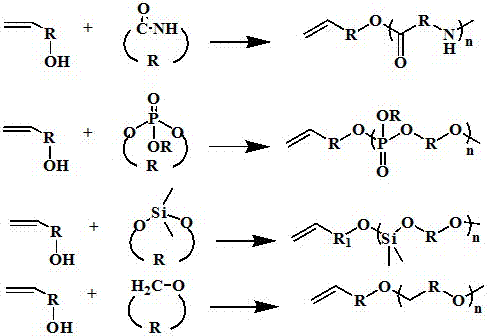
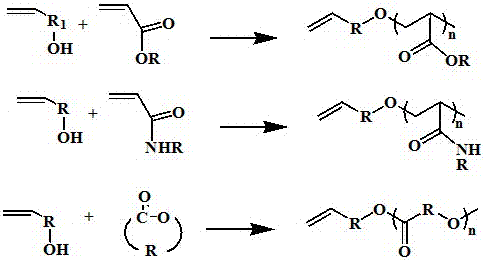
A kind of synthetic method of macromonomer
A technology of macromonomer and synthesis method, which is applied in the field of macromonomer preparation, and achieves the effects of rich structure, mild reaction conditions and broadened application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 0.34 g (3 mmol) caprolactone, 0.15 g (0.3 mmol) p-vinylbenzyl alcohol, and 0.1 g THF to a dry 50 mL reaction flask. The reaction system, after three times of "vacuumizing-nitrogen", added 300 mu L (0.3 mmol) t -BuP 4 n-hexane solution. at -30 o After reacting for 48 h under the condition of C, 2 mL of 10 wt% hydrochloric acid solution was added dropwise to terminate the above reaction. Then use 2 × 100 mL methanol to fully wash the polymer under rapid stirring to remove unreacted monomer and solvent, filter, 50 o C under vacuum for 12 h, the calculated yield was 80%, and the molecular weight was 1500 g / mol.
Embodiment 2
[0031] Add 34 g (300 mmol) of caprolactone and 0.15 g (0.3 mmol) of p-vinylbenzyl alcohol into a dry 250 mL reaction flask. The reaction system, after three times of "vacuumizing-nitrogen", added 300 mu L (0.3mmol) t -BuP 4 n-hexane solution. at 200 o After reacting at C for 0.5 h, 2 mL of 10 wt% hydrochloric acid solution was added dropwise to terminate the above reaction, and 50 mL of THF was added to dissolve the polymer. Then use 3 × 200 mL methanol to fully wash the polymer under rapid stirring to remove unreacted monomers, filter, 50 o C under vacuum for 12 h, the calculated yield was 84%, and the molecular weight was 134200 g / mol.
Embodiment 3
[0033] Add 0.34 g (3 mmol) caprolactone, 0.15 g (0.3 mmol) p-vinylbenzyl alcohol, and 1.0 g THF to a dry 50 mL reaction flask. The reaction system, after three times of "vacuumizing-nitrogen", added 0.01g (0.3 mmol) NaOH solution in n-hexane under the protection of nitrogen. at 25 o After reacting for 48 h under the condition of C, 2 mL of 10 wt% hydrochloric acid solution was added dropwise to terminate the above reaction. at -30 o After 48 h of reaction under C conditions, 2 mL of 10 wt% hydrochloric acid solution was added dropwise to terminate the above reaction. Then use 2 × 100 mL methanol to fully wash the polymer under rapid stirring to remove unreacted monomers and solvents, filter, 50 o C under vacuum for 12 h, the calculated yield was 60%, and the molecular weight was 1600 g / mol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com