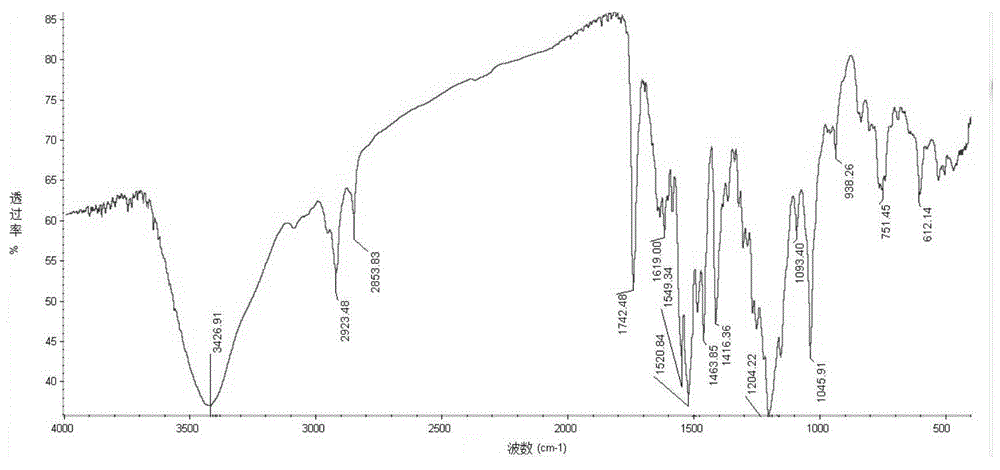
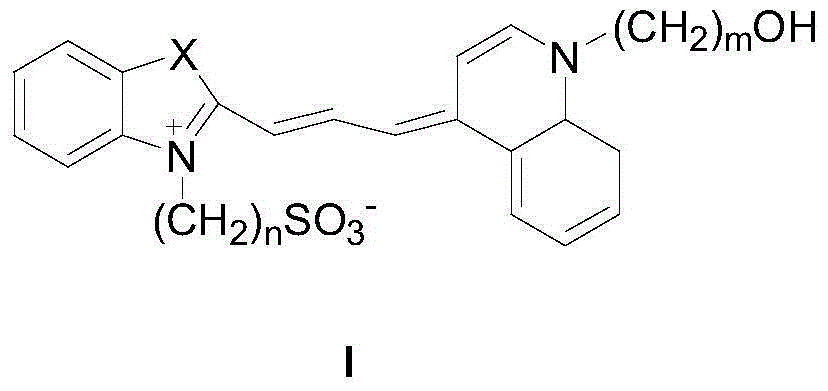
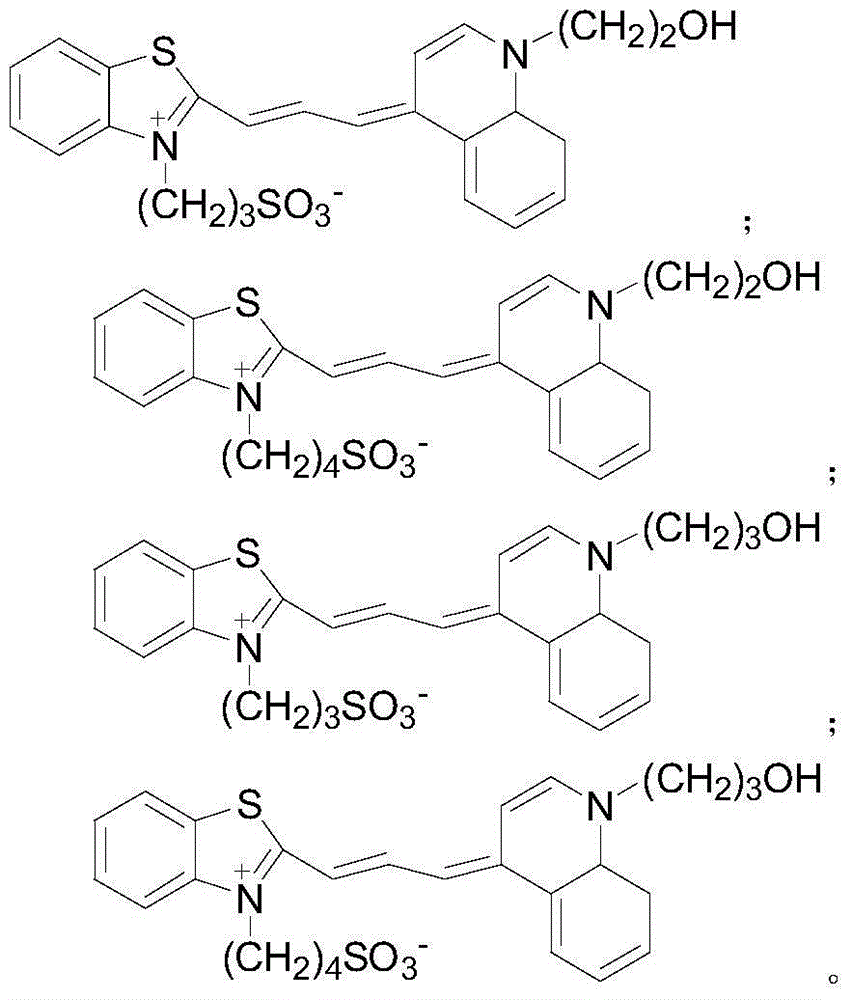
Fluorochrome compound as well as preparation method and application thereof
A technology of fluorescent dyes and compounds, applied in the field of fine chemicals, can solve the problems of impermeability, complex structure, and large molecules of living cells, and achieve the effects of good cell membrane permeability, high sensitivity, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] preparation of
[0026] 1) The first quaternary ammonium salt intermediate III (n=3) is obtained by reacting the benzothiazole aromatic heterocyclic compound of the formula II compound with propane sultone; the reaction temperature is 150°C, the reaction time is 20 hours, and the reaction solvent Selected from: dichloromethane, chloroform, the molar ratio of formula II compound and propane sultone or butane sultone is 1:1;
[0027] 2) Condensing the first quaternary ammonium salt intermediate III with N,N,-dibenzamidine to obtain the compound of formula IV; the reaction temperature is 150°C, the reaction time is 24 hours, the reaction solvent is acetic anhydride, and acetic acid is a protonic acid Catalyst, the mol ratio of the first quaternary ammonium salt and benzamidine is 1:3;
[0028] 3) Reaction of compound IV with compound of formula V (m=2) to obtain compound of formula VI: the reaction temperature is 150°C, the reaction time is 24 hours, the reaction solven...
Embodiment 2
[0033] preparation of
[0034] 1) The first quaternary ammonium salt intermediate III (n=4) is obtained by reacting the benzothiazole aromatic heterocyclic compound of the formula II compound with butane sultone; the reaction temperature is 200°C, the reaction time is 10 hours, and the reaction solvent Selected from: ethanol, acetonitrile, the molar ratio of formula II compound and propane sultone or butane sultone is 1:3;
[0035] 2) Condensing the first quaternary ammonium salt intermediate III with N,N,-dibenzamidine to obtain the compound of formula IV; the reaction temperature is 50°C, the reaction time is 20 hours, the reaction solvent is acetic anhydride, and acetic acid is a protonic acid Catalyst, the mol ratio of the first quaternary ammonium salt and benzamidine is 1:2;
[0036] 3) Reaction of compound IV with compound of formula V (m=2) to obtain compound of formula VI: the reaction temperature is 10°C, the reaction time is 48 hours, the reaction solvent is acet...
Embodiment 3
[0038] preparation of
[0039] 1) The first quaternary ammonium salt intermediate III (n=3) is obtained by reacting the benzothiazole aromatic heterocyclic compound of the formula II compound with propane sultone; the reaction temperature is 10°C, the reaction time is 48 hours, and the reaction solvent Selected from: ethyl acetate, toluene, the molar ratio of the compound of formula II to propane sultone or butane sultone is 1:5;
[0040] 2) Condensing the first quaternary ammonium salt intermediate III with N,N,-dibenzamidine to obtain the compound of formula IV; the reaction temperature is 100°C, the reaction time is 10 hours, the reaction solvent is acetic anhydride, and acetic acid is a protonic acid Catalyst, the mol ratio of the first quaternary ammonium salt and benzamidine is 1:1;
[0041] 3) Reaction of compound IV with compound of formula V (m=3) to obtain compound of formula VI: the reaction temperature is 85°C, the reaction time is 24 hours, the reaction solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com