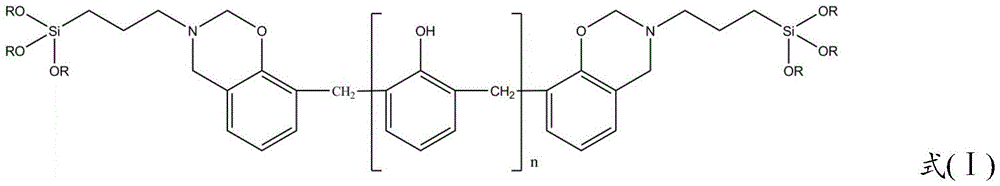
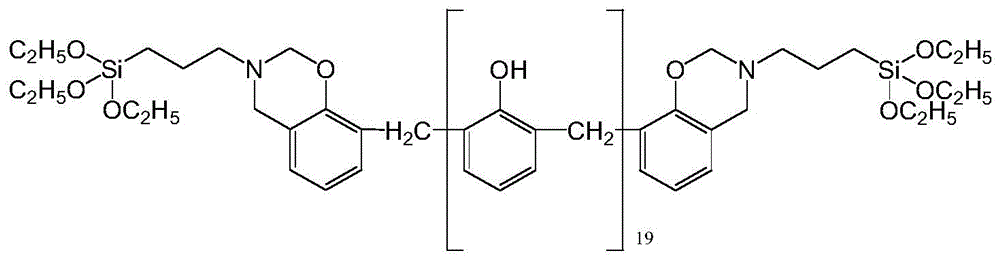
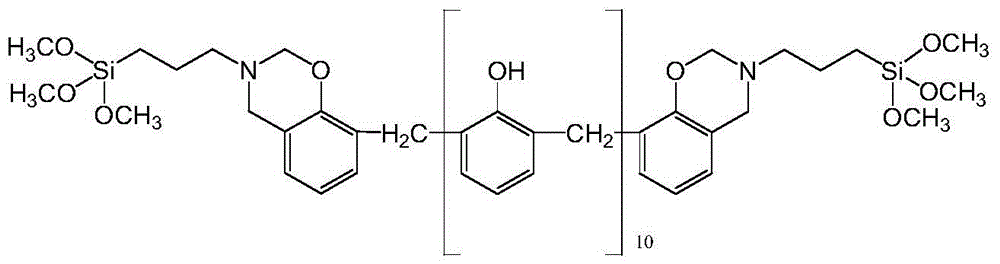
Silane-modified phenolic resin and preparation method thereof
The technology of novolac resin and phenolic resin is applied in the field of silane-modified phenolic resin and its preparation. efficiency, overcoming the effect of uneven blending and dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of the silane-modified phenolic resin of the present embodiment may further comprise the steps:
[0033] (1), preparation novolac resin
[0034] Add 188.015g (2mol) of phenol, 137.062g of 37wt% formaldehyde solution (1.7mol), 100g of distilled water, and 1.88g of oxalic acid (as a catalyst) to a 1000ml three-necked flask. React at 125°C for half an hour. After completion of the reaction, the product was poured into distilled water and washed repeatedly to remove unreacted free phenol and formaldehyde, and the catalyst, and further remove small molecules in vacuum to obtain a translucent viscous fluid with a viscosity of 2100cp, which is novolac resin (203.179g ), hydroxyl equivalent 107, molecular weight Mn=1560, molecular weight distribution d=1.67;
[0035] (2), prepare Schiff base trialkoxysilane
[0036] 44.231g γ-aminopropyltriethoxysilane (0.2mol), 12.017g paraformaldehyde (0.4mol), 150ml chloroform, and 10g CaH 2 , reacted under vigorou...
Embodiment 2
[0050] The preparation method of the silane-modified phenolic resin of the present embodiment may further comprise the steps:
[0051] (1), preparation novolac resin
[0052] Add 94.067g (1mol) of phenol, 64.916g of 37wt% formaldehyde solution (0.8mol), 50g of distilled water, and 0.9516g of oxalic acid into a 500m three-necked flask. half an hour. After completion of the reaction, the product was poured into distilled water and washed repeatedly to remove unreacted free phenol and formaldehyde, and the catalyst, and further remove small molecules in vacuum to obtain a translucent viscous fluid with a viscosity of 700 cp, which is novolac resin (102.601g ), hydroxyl equivalent 109, molecular weight Mn=950, molecular weight distribution d=1.27;
[0053] (2), prepare Schiff base trialkoxysilane
[0054] 35.858g γ-aminopropyltrimethoxysilane (0.2mol), 12.017g paraformaldehyde (0.4mol), 150ml toluene, and 10g CaH 2 , reacted under vigorous stirring in a 500ml three-necked flas...
Embodiment 3
[0068] The preparation method of the silane-modified phenolic resin of the present embodiment may further comprise the steps:
[0069] (1), preparation novolak resin
[0070] Add 376.268g (4mol) of phenol, 292.013g of 37wt% formaldehyde solution (3.598mol), 50g of distilled water, and 3.636g of oxalic acid to a 2000m three-necked flask. half an hour. After completion of the reaction, the product was poured into distilled water and washed repeatedly to remove unreacted free phenol and formaldehyde, and the catalyst, and further remove small molecules in vacuum to obtain a translucent viscous fluid with a viscosity of 5100 cp, which is novolac resin (418.528g ), hydroxyl equivalent 108, molecular weight Mn=3700, molecular weight distribution d=1.57;
[0071] (2), prepare Schiff base trialkoxysilane
[0072] 44.206g γ-aminopropyltriethoxysilane (0.2mol), 12.099g paraformaldehyde (0.2mol), 150ml dichloromethane, and 15g CaH 2 , reacted under vigorous stirring in a 1000ml three...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com