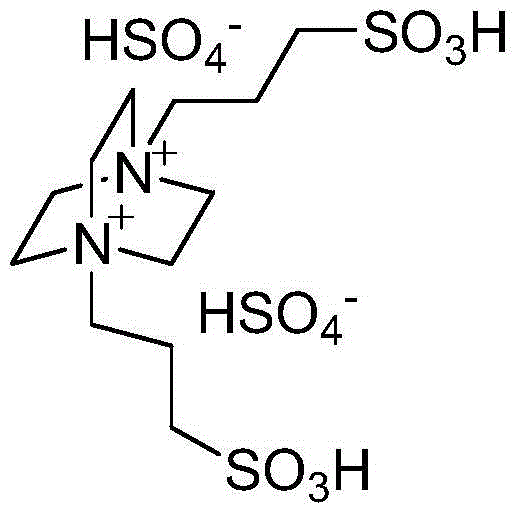
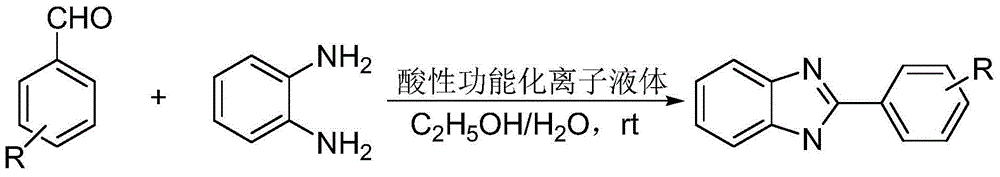
Method for catalytic synthesis of benzimidazole derivatives
A technology for benzimidazole derivatives and derivatives, which is applied in the field of catalytic synthesis of benzimidazole derivatives, can solve the problems of large usage and loss, complicated preparation process, difficult biodegradation, etc. Simple, inexpensive ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 1mmol of benzaldehyde, 1mmol of o-phenylenediamine and 0.03mmol of degradable acidic ionic liquid catalyst to 4ml of water and ethanol mixture (V (water): V (ethanol)=1:1) with stirring Stir vigorously at room temperature for 7 minutes, followed by TLC (thin plate chromatography) detection. After the reaction, a large amount of solids precipitate out, crush the solids, let stand, and filter with suction. The resulting filter residue is vacuum-dried to obtain pure 2-benzene Base-1H-benzimidazole with a yield of 98%. The filtrate was directly added with benzaldehyde and o-phenylenediamine for repeated use.
[0024] 2-Phenyl-1H-benzimidazole: m.p.285~287℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.18~7.27(m, 2H), 7.45~7.56(m, 4H), 7.66~7.77(m, 1H), 8.15~8.23(m, 2H), 12.94(s, 1H)
Embodiment 2
[0026] Add 1mmol of p-chlorobenzaldehyde, 1mmol of o-phenylenediamine and 0.04mmol of degradable acidic ionic liquid catalyst to the belt containing 5ml of water and ethanol mixture (V (water): V (ethanol) = 1:1). In a 25ml single-necked bottle with a stirrer, stir vigorously at room temperature for 8 minutes, TLC (thin-plate chromatography) tracking detection, after the reaction is completed, a large amount of solids are precipitated, crushed solids, left standing, and suction filtered, and the obtained filter residue is vacuum-dried to obtain pure 2 -(4-Chlorophenyl)-1H-benzimidazole, the yield was 98%. After directly adding p-chlorobenzaldehyde and o-phenylenediamine in the filtrate, it is reused.
[0027] 2-(4-Chlorophenyl)-1H-benzimidazole: m.p.291~292℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.12~7.31(m, 2H), 7.46~7.73(m, 4H), 8.17(d, J=8.6Hz, 2H), 12.98(s, 1H)
Embodiment 3
[0029]Add 1mmol of m-nitrobenzaldehyde, 1mmol of o-phenylenediamine and 0.05mmol of degradable acidic ionic liquid catalyst to the tank containing 5ml of water and ethanol mixed solution (V (water): V (ethanol) = 1:1). In a 25ml single-necked bottle with a stirrer, stir vigorously at room temperature for 26min, TLC (thin plate chromatography) tracking detection, after the completion of the reaction, a large amount of solids precipitate out, crush the solids, stand still, and suction filter, the obtained filter residue is vacuum-dried to obtain pure 2-(3-nitrophenyl)-1H-benzimidazole, yield 97%. After directly adding m-nitrobenzaldehyde and o-phenylenediamine in the filtrate, it is reused.
[0030] 2-(3-nitrophenyl)-1H-benzimidazole: m.p.213~214℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.29(s, 2H), 7.61(d, J=6.0Hz, 1H), 7.74(d, J=6.9Hz, 1H), 7.87(t, J=8.0Hz, 1H), 8.60~8.65 (m, 1H), 9.02~9.06(m, 1H), 13.28(s, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com