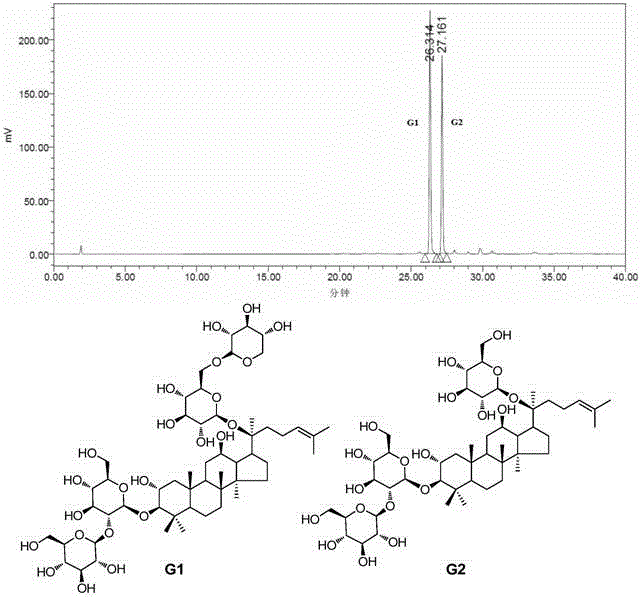
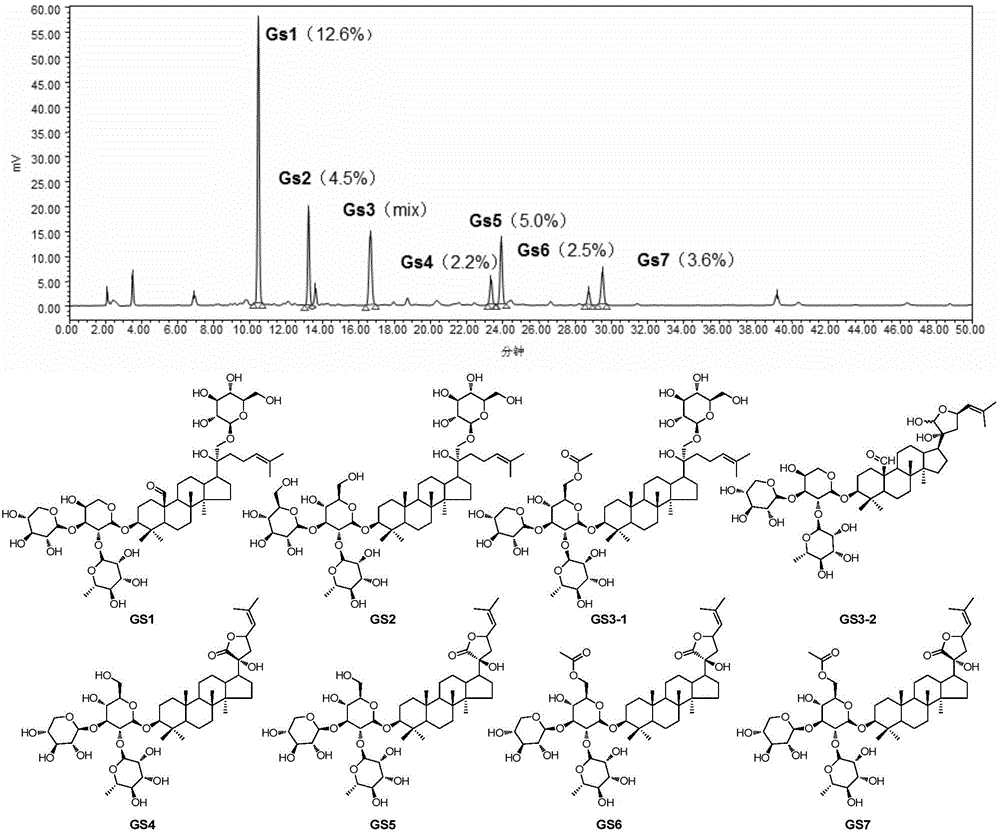
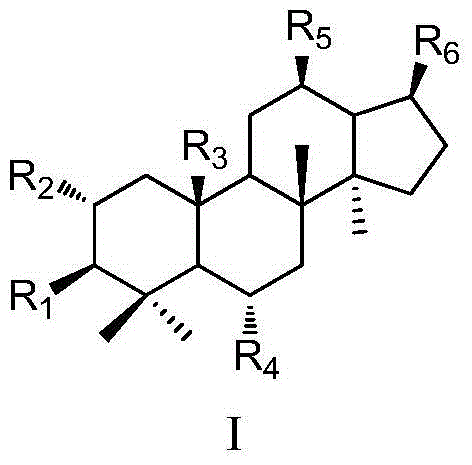
Use of dammarane-type triterpene derivatives
A technology of use and alkyl, applied in the field of use of dammarane-type triterpenoid derivatives, can solve the problem of less AMPK direct agonists and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 compound 1
[0050]
[0051] In a 2L three-necked reaction flask, add 100g of American ginseng total saponins (purchased from Fusong Nature Bioengineering Co., Ltd.) and 1300ml of n-butanol, and stir for 10 minutes, then add 40g of sodium metal in batches, stir until no hydrogen is released, and then add 10 g of benzoyl peroxide was fed into oxygen, and heated to 120° C. for 24 hours. After the TLC detection reaction was complete, the reaction solution was cooled to room temperature, and n-butanol was removed under reduced pressure, dissolved in water, then extracted with petroleum ether and ethyl acetate in sequence, and the ethyl acetate part was distilled under reduced pressure to obtain an extract containing compound 1 , after silica gel column chromatography (petroleum ether: ethyl acetate = 2:1 (volume ratio) elution), compound 1 (7g, 7%) was obtained. 1 H NMR (300MHz, CDCl 3 )δ5.19(t,J=7.3Hz,1H),3.63(td,J=10.4,5.1Hz,1H),3.22(d...
Embodiment 2
[0052] The preparation of embodiment 2 compound 2
[0053]
[0054] In a 50ml three-neck reaction flask, dissolve compound 1 (200mg, 0.435mmol) in 15ml CH 2 Cl 2 After stirring for 10 min at -78°C, O 3 Reaction 2min. Then warm up to -3°C, add methylamine (0.1ml), NaBH(OAc) 3 (368.8mg, 1.7mmol) and CH 3 OH (8ml), stir overnight, after the reaction is complete, add 15ml of water, 2 Cl 2 (30ml) extracted twice, dried over anhydrous sodium sulfate, concentrated CH under reduced pressure 2 Cl 2 , Compound 2 was obtained by silica gel column chromatography (dichloromethane / methanol / triethylamine, 40:1:0.5 (volume ratio)), with a yield of 85%. 1 H NMR (300MHz, CDCl 3 )δ4.12(m,1H),3.52(td,J=13.0,6.2Hz,1H),3.21(dd,J=10.9,5.0Hz,1H),2.94(m,1H),2.62(m,1H ),2.47(s,3H),1.13(s,3H),0.99(s,3H),0.98(s,3H),0.88(s,6H),0.78(s,3H),0.73(d,J= 11.0Hz,1H);ESI-MS450.7[M+H] + .
Embodiment 3
[0055] The preparation of embodiment 3 compound 3
[0056]
[0057] Except replacing methylamine with ethylamine, compound 3 is prepared in the same manner as in Example 2; Silica gel column chromatography (dichloromethane / methanol / triethylamine, 45:1:0.5 (volume ratio)), the yield is 86%. 1 H NMR (300MHz, CDCl 3 )δ3.55(td,J=12.9,6.3Hz,1H),3.19(dd,J=10.9,4.9Hz,1H),2.93(m,1H),2.72(m,1H),2.58(m,1H ),2.48(m,1H),2.09(m,1H),1.15(s,3H),1.11(t,J=7.2Hz,3H),0.99(s,3H),0.98(s,3H),0.89 (s,3H),0.88(s,3H),0.78(s,3H),0.73(d,J=10.7Hz,1H);ESI-MS464.7[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com