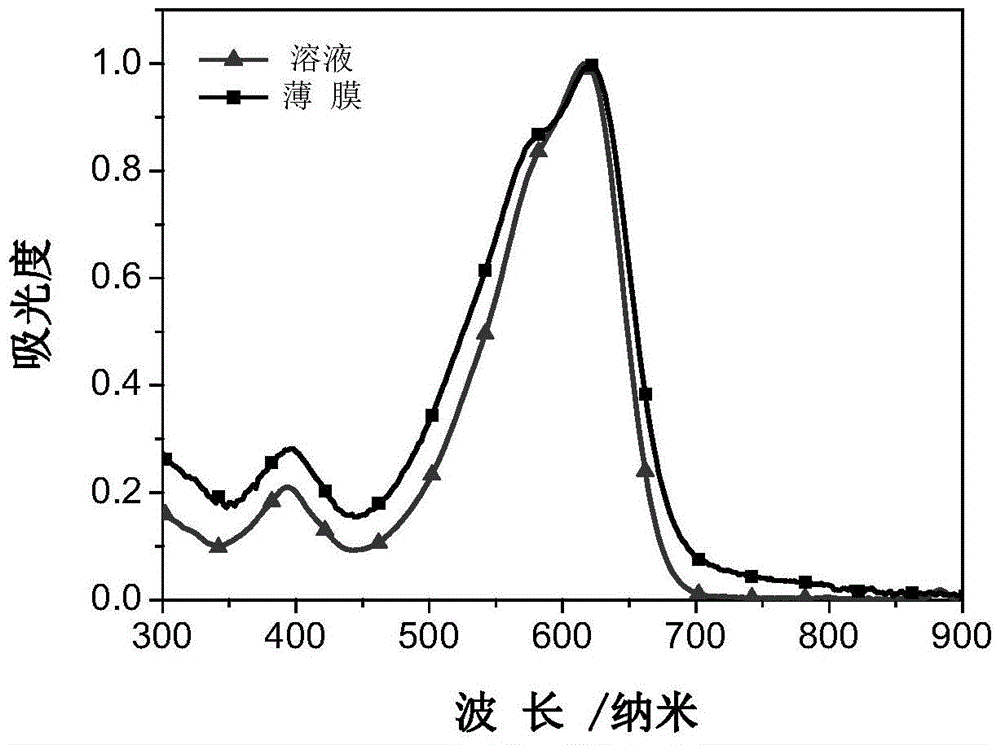
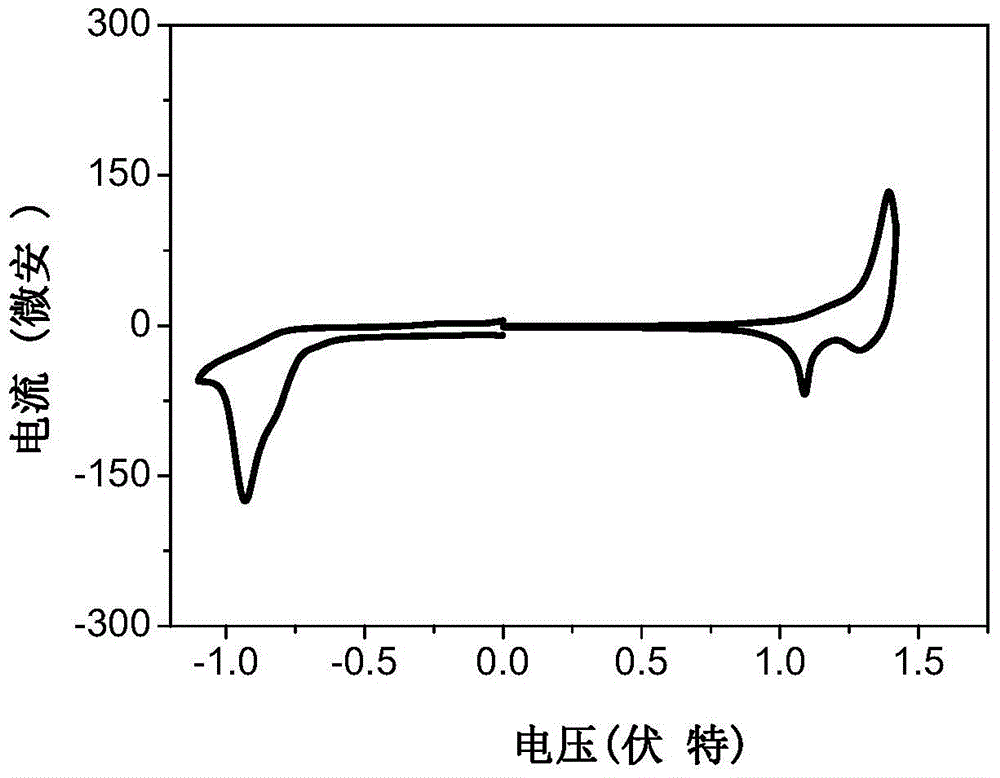
A-d-a conjugated molecule based on dithienoindacene and its preparation method and application
A technology of conjugated molecule and dithiophene, which is applied in the field of A-D-A conjugated molecule based on dithiophene and indacene and its preparation and application, to achieve good thermal stability, good light absorption and excellent thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The synthetic route of A-D-A conjugated molecule 1 based on dithienoindacene is as follows:
[0084]
[0085] Take 246mg (0.20mmol) of compound a and 159.5mg (0.50mmol) of compound b in a 50mL three-necked flask, add 25mL of freshly distilled anhydrous toluene; under stirring, pass argon for 15 minutes to remove the air in the three-necked flask, then Under the protection of Pd(PPh 3 ) 4 (40mg, 0.034mmol), heated to 110°C, refluxed for 24 hours; after cooling to room temperature, add 10mL (0.1g mL -1 ) KF aqueous solution, stirred overnight at room temperature to remove tin impurities; add 100mL water, CHCl 3 (2 × 100mL) extraction; organic phase with anhydrous MgSO 4 Carry out drying; After filtering and spinning to remove the solvent, use petroleum ether / dichloromethane mixed solvent (volume ratio 1:2) as eluent, silica gel (200~300 mesh) column chromatography separation and purification, obtain black solid (248mg , 89.7%), which is the A-D-A conjugated molecul...
Embodiment 2
[0088] The synthetic route of A-D-A conjugated molecule 2 based on dithienoindacene is as follows:
[0089]
[0090] Take 492mg (0.40mmol) of compound a, 302mg (1.0mmol) of compound c in a 50mL three-necked flask, add 30mL of freshly distilled anhydrous toluene; under stirring, pass argon for 15 minutes to remove the air in the three-necked flask, and then Under protection, adding Pd(PPh 3 ) 4 (40mg, 0.034mmol), heated to 110°C, refluxed for 24 hours; after cooling to room temperature, add 10mL (0.1gmL -1 ) KF aqueous solution, stirred overnight at room temperature to remove tin impurities; add 100mL water to the reactant, CH 2 Cl 2 (2 × 100mL) extraction; organic phase with anhydrous MgSO 4 Carry out drying; After filtering and spinning to remove the solvent, use petroleum ether / dichloromethane mixed solvent (volume ratio 1:4) as eluent, silica gel (200~300 mesh) column chromatography separation and purification to obtain a black solid (181mg , 33.5%), which is the A-...
Embodiment 3
[0093] That is, the synthetic route of A-D-A conjugated molecule 3 based on dithienoindacene is as follows:
[0094]
[0095] Take 221.8mg (0.18mmol) of compound a and 165mg (0.45mmol) of compound d in a 50mL three-necked flask, add 20mL of freshly distilled anhydrous toluene; under stirring, pass argon for 15 minutes to remove the air in the three-necked flask, then Under the protection of Pd(PPh 3 ) 4 (40mg, 0.034mmol), heated to 110°C, refluxed for 24 hours; after cooling to room temperature, add 40mL (0.1g mL -1 ) KF aqueous solution, stirred overnight at room temperature to remove tin impurities; add 100mL water, CHCl 3 (2 × 100mL) extraction; organic phase with anhydrous MgSO 4 Carry out drying; After filtering and spinning to remove the solvent, use petroleum ether / dichloromethane mixed solvent (volume ratio 1:4) as eluent, silica gel (200~300 mesh) column chromatography separation and purification to obtain a black solid (230mg , 86.4%), which is the A-D-A conju...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com