Bimetallic organic gel and preparation and application thereof in detection of cyanide ions
An organogel, bimetallic technology, applied in the field of anion detection, can solve problems such as large demand and no supramolecular gel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 5.0 mmol of 3,4,5-tris(hexadecyloxy)benzoic hydrazide, 5.0 mmol of 1-naphthaldehyde and 30 mL of absolute ethanol (as solvent) to a 50 mL reaction bottle, and 0.12~0.24 mL of ice Acetic acid (catalyst), reflux and stir for 8h, suction filter after cooling to obtain a white solid; recrystallize with chloroform-ethanol to obtain gel factor G .
[0029]Weigh 10 mg (0.01 mmol) of gelling factor G and add it into 1 mL of ethanol, heat it to dissolve, and form a white organic gel OG after cooling to room temperature (the mass percentage of gelling factor is 1%). Organogel OG is non-fluorescent. Then add 0.01 mmol of Mg 2+ ethanol solution to make it diffuse slowly; after standing for a period of time, Mg 2+ The ethanol solution completely infiltrates into the gel, which is the magnesium metal organogel MgG, and it is relatively stable. The magnesium metal organogel has a strong bright blue fluorescence; then add 0.01 mmol Cu to it 2+ ethanol solution to make it diffus...
Embodiment 2
[0032] The preparation of gel factor G is the same as in Example 1.
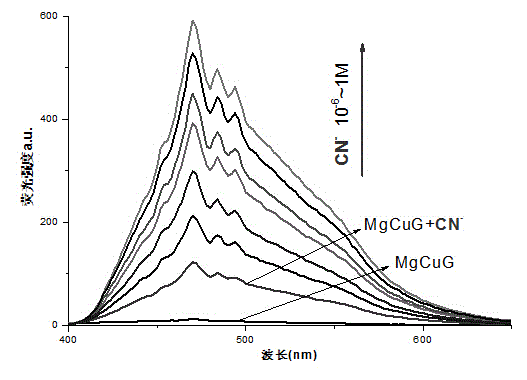
[0033] Weigh 10 mg (0.01 mmol) of gelling factor G and add it into 1 mL of ethanol, heat it to dissolve, and form a white organic gel OG after cooling to room temperature (the mass percentage of gelling factor is 1%). Organogel OG has no fluorescence under the irradiation of 365nm ultraviolet lamp. Then add (0.02 mmol) Mg 2+ and (0.02 mmol) Cu 2+ The ethanol solution was shaken to disperse evenly, and the magnesium-copper bimetallic organogel MgCuG was formed after cooling to room temperature. The organogel MgCuG has no fluorescence under the irradiation of 365nm ultraviolet lamp.
[0034] Addition of CN to bimetallic organogel MgCuG - In aqueous solution, MgCuG gel produced strong bright blue fluorescence, while F - , Cl - , Br - , I - , AcO - , H 2 PO 4 - , N 3 - , SCN - , HSO 4 - , ClO 4 - The addition of isoanionic aqueous solution cannot produce similar fluorescence change phenomenon....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com