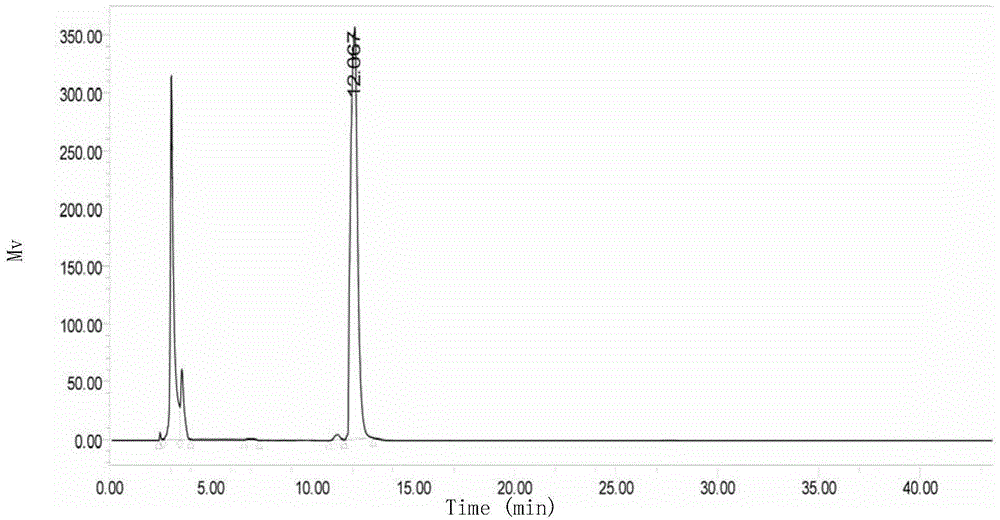
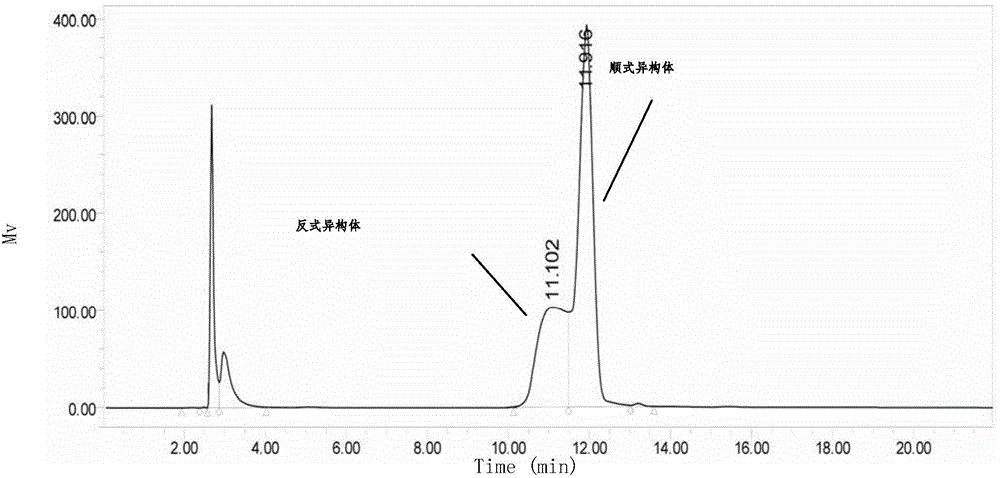
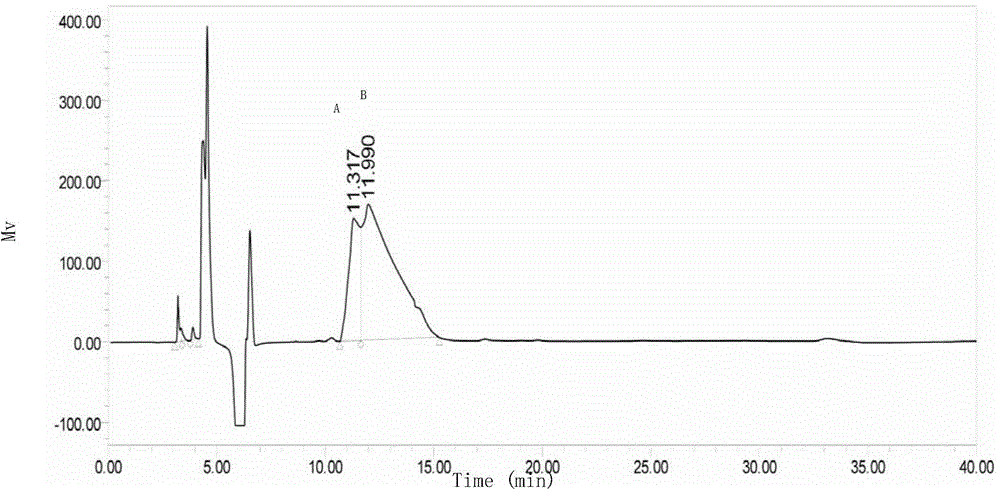
Production method for cyclic dipeptide
A production method and cyclic dipeptide technology, which is applied in the field of synthesis of natural compounds, can solve the problems of CHP efficacy weakening, disappearance, slow reaction rate, etc., and achieve the effect of inhibiting racemization reaction, easy to master, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Low temperature protection of amino acid amino group
[0029] Using L-histidine as raw material, dissolve it in water-tetrahydrofuran (v / v=1:2) solvent, the concentration of L-histidine is 0.25mol / L, di-tert-butyl dicarbonate [( Boc) 2 O] carry out amino protection for protecting agent, first prepare tetrahydrofuran-(Boc) 2 O solution, where (Boc) 2 The concentration of O is 1mol / L, add to the alkali aqueous solution of pH 8 containing L-histidine, His: (Boc) 2 O(mol / mol)=1:2, Stir the reaction at -5°C for 15min, the stirring rate is 80r / min, then raise the temperature to 30°C and stir the reaction for 4h, after the reaction is completed, the temperature is 38°C and the vacuum degree is -0.1MPa. The organic solvent was removed under reduced pressure, the residue was dissolved in water at a ratio of 1:18 (g / mL), washed twice with ether, and the water phase / ether phase (v / v)=2:1 during each washing, to remove the residual (Boc) 2 O, then adjusted to pH 3 with 50%...
Embodiment 2
[0039] (1) Low temperature protection of amino acid amino group
[0040] Using L-histidine as raw material, dissolve it in water-tetrahydrofuran (v / v=1:2) solvent, the concentration of L-histidine is 0.50mol / L, and di-tert-butyl dicarbonate [( Boc) 2 O] carry out amino protection for protecting agent, first prepare tetrahydrofuran-(Boc) 2 O solution, where (Boc) 2 The concentration of O is 1.5mol / L, added to the alkaline aqueous solution of pH 9 containing L-histidine, His: (Boc) 2 O(mol / mol)=1:2.5, stir the reaction at -10°C for 30min, the stirring rate is 100r / min, then raise the temperature to 40°C and stir the reaction for 6h, after the reaction is completed, the temperature is 38°C and the vacuum degree is -0.1MPa. The organic solvent was removed under reduced pressure, and the residue was dissolved in water at a ratio of 1:20 (g / mL), washed with ether three times, and the water phase / ether phase (v / v)=2:1 during each washing, to remove the residual (Boc) 2 O, and th...
Embodiment 3
[0050] (1) Low temperature protection of amino acid amino group
[0051] Using L-histidine as raw material, dissolve it in water-tetrahydrofuran (v / v=1:2) solvent, the concentration of L-histidine is 0.38mol / L, and di-tert-butyl dicarbonate [( Boc) 2 O] carry out amino protection for protecting agent, first prepare tetrahydrofuran-(Boc) 2 O solution, where (Boc) 2 The concentration of O is 1.25mol / L, added to the alkaline aqueous solution of pH 8.5 containing L-histidine, His: (Boc) 2 O(mol / mol)=1:2.2. Stir the reaction at -8°C for 25min, the stirring rate is 90r / min, then raise the temperature to 35°C and stir the reaction for 5h. After the reaction, remove the organic solvent under reduced pressure at a temperature of 38°C and a vacuum of -0.1MPa, and the residue 1: 19 (g / mL) ratio was added to dissolve in water, washed 3 times with ether, water phase / ether phase (v / v) = 2: 1 during each wash, to remove residual (Boc) 2 O, then adjusted to pH 3.5 with 50% citric acid so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com