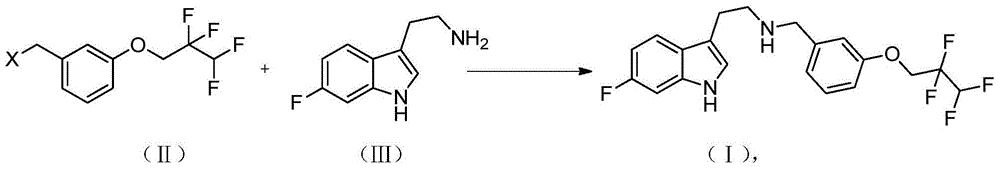
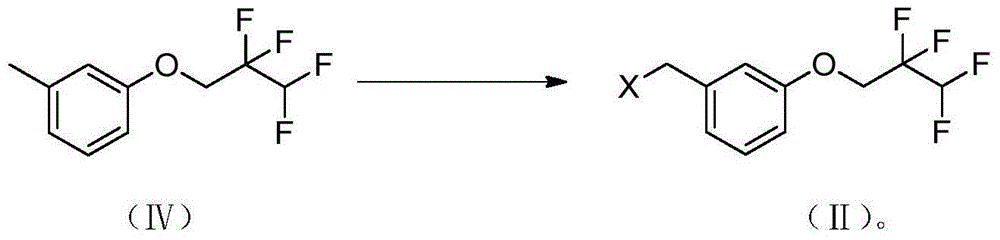
Method for preparing medicine Lu-AE-58054 for resisting alzheimer's disease
A compound and solvent technology, applied in the field of preparation of anti-Alzheimer's drug Lu-AE-58054, can solve the problems of high energy consumption and high price of 3-hydroxybenzaldehyde
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 2,2,3,3-tetrafluoropropoxy-4-methylbenzenesulfonate
[0081] 2,2,3,3-Tetrafluoro-1-propanol (3.0 g, 22.7 mmol) and triethylamine (6.4 mL, 45.4 mmol) were dissolved in dichloromethane (20 mL), and then slowly added in portions to Toluenesulfonyl chloride (2.87g, 15.0mmol), after reacting at room temperature for 6 hours, dichloromethane (50ml) was added to dilute, washed with saturated sodium chloride solution (50mL), and the organic phase was dried with anhydrous sodium sulfate after separation. After filtration, the filtrate was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=20 / 1) to obtain the title compound as a colorless liquid (3.67 g, 85%).
[0082] 1 H NMR (600MHz, CDCl 3 )δ (ppm): 7.83 (d, J = 8.2Hz, 2H), 7.41 (d, J = 8.1Hz, 2H), 5.88 (tt, J = 52.9, 4.1Hz, 1H), 4.36 (t, J = 12.0Hz, 2H), 2.50(s, 3H).
Embodiment 2
[0083] Example 2 1-methyl-3-(2,2,3,3-tetrafluoropropoxy)benzene
[0084] 2,2,3,3-Tetrafluoropropoxy-4-methylbenzenesulfonate (572 mg, 2 mmol) was dissolved in N,N-dimethylformamide (10 mL), and potassium carbonate was added thereto (276mg, 2mmol) and m-cresol (432mg, 4mmol), heated to 100°C for reaction, monitored by TLC. After the reaction was complete, saturated brine (30 mL) was added, and extracted with ethyl acetate (30 mL x 3). The combined organic phases were dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=100 / 1) to obtain the title compound as white Solid (328 mg, 74%).
[0085] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 7.23 (t, J = 7.8Hz, 1H), 6.89 (d, J = 7.5Hz, 1H), 6.78 (s, 1H), 6.75 (d, J = 8.2Hz, 1H), 6.08 (tt, J=53.1, 5.0Hz, 1H), 4.35(t, J=11.9Hz, 2H), 2.37(s, 3H).
Embodiment 3
[0086] Example 3 1-(bromomethyl)-3-(2,2,3,3-tetrafluoropropoxy)benzene
[0087] 1-Methyl-3-(2,2,3,3-tetrafluoropropoxy)benzene (444 mg, 2 mmol) was dissolved in carbon tetrachloride (10 mL), and azobisisobutyronitrile was added thereto (10mg, 0.06mmol) and N-bromosuccinimide (356mg, 2mmol), heated to 80°C for reaction, monitored by TLC. After the reaction was completed, it was filtered and the filtrate was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=50 / 1) to obtain the title compound as a white solid (450 mg, 75%).
[0088] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 7.22 (t, J = 7.9Hz, 1H), 7.00 (d, J = 7.5Hz, 1H), 6.90 (s, 1H), 6.79 (dd, J = 8.2, 2.0Hz, 1H) , 5.99 (tt, J=52.8, 4.9Hz, 1H), 4.38(s, 2H), 4.28 (t, J=11.8Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com