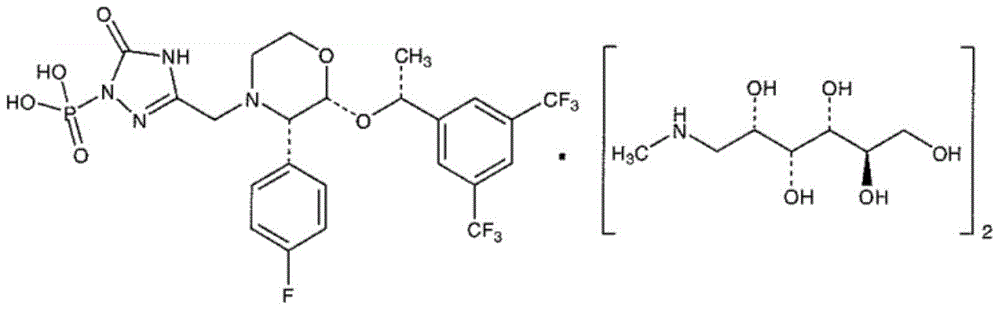
A kind of fosaprepitant dimeglumine composition for injection and preparation method thereof
A technology of fosaprepitant dimeglumine and its composition, which is applied in the direction of drug combination, medical preparations without active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of decreased blood calcium concentration and lactose intolerance Symptoms, potential safety hazards, etc., to achieve strong solubilization ability, eliminate blood calcium concentration drop, and use safely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
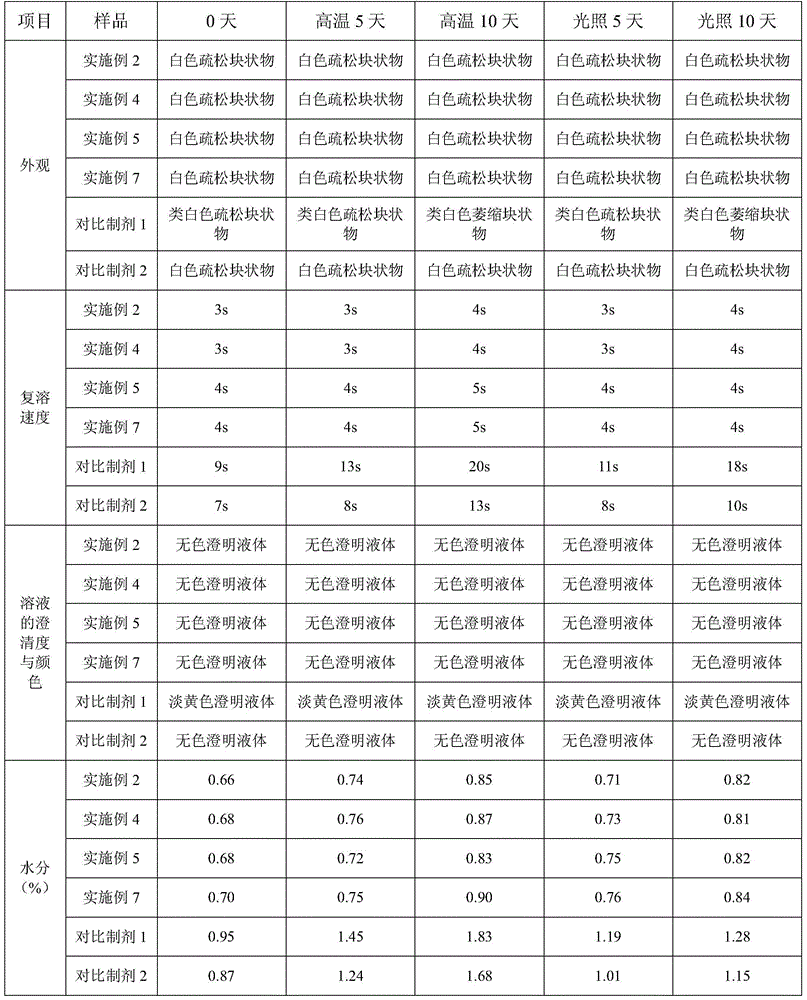
Examples
Embodiment 1
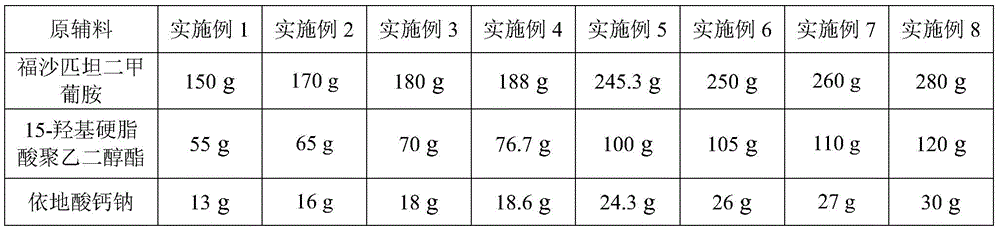
[0038] Preparation process: inject 2100ml of water for injection into the liquid mixing tank, the temperature of the water for injection is within the range of 20°C to 30°C, first add 55g of polyethylene glycol 15-hydroxystearate and 13g of calcium sodium edetate to dissolve Finally, add 150 g of fosaprepitant dimeglumine, stir to dissolve it completely, adjust the pH to 8.0 with 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution, add water for injection to 3000ml, stir evenly, and add the prepared total Measure 0.1% activated carbon for needles, stir and let it stand for 20 minutes, filter and sterilize the liquid medicine with a 0.22μm+0.22μm cartridge filter; fill and half-tamp; the product is stored at -20°C in a freeze dryer drying oven Pre-freeze for 4 hours, vacuumize the box, dry at a vacuum degree of 9-13 Pa, and a temperature of -20°C to 0°C for 10 hours, keep the vacuum condition at 0°C-15°C for 5 hours, and dry at 20°C Dry under the conditions fo...
Embodiment 2
[0040] Preparation process: inject 2500ml of water for injection into the liquid mixing tank, the temperature of the water for injection is within the range of 20°C to 30°C, first add 65g of polyethylene glycol 15-hydroxystearate and 16g of calcium sodium edetate to dissolve Finally, add 170g of fosaprepitant dimeglumine, stir to dissolve it completely, adjust the pH to 8.2 with 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution, add water for injection to 3000ml, stir evenly, and add the prepared total Measure 0.1% activated carbon for needles, stir and let it stand for 20 minutes, filter and sterilize the medicinal solution with a 0.22μm+0.22μm cartridge filter; fill and half-tamp; the product is stored at -30°C in a freeze dryer drying oven Pre-freeze for 4 hours, vacuumize the box, dry for 13 hours at a vacuum degree of 9 to 13 Pa, and a temperature of -20°C to 0°C, keep the vacuum condition at 0°C to 15°C for 6 hours, and dry at 25°C Dry under condition...
Embodiment 3
[0042] Preparation process: Inject 2500ml of water for injection into the liquid mixing tank. The temperature of the water for injection is within the range of 20°C to 30°C. First, add 70g of polyethylene glycol 15-hydroxystearate and 18g of calcium sodium edetate to dissolve Finally, add 180 g of fosaprepitant dimeglumine, stir to dissolve it completely, adjust the pH to 8.2 with 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide solution, add water for injection to 3000ml, stir evenly, and add the prepared total Measure 0.1% activated carbon for needles, stir and let it stand for 20 minutes, filter and sterilize the medicinal solution with a 0.22μm+0.22μm cartridge filter; fill and half-tamp; the product is stored at -30°C in a freeze dryer drying oven Pre-freeze for 3 hours, vacuumize the box, dry at a vacuum degree of 9-13 Pa, and a temperature of -20°C to 0°C for 14 hours, keep the vacuum condition at 0°C-15°C for 7 hours, and dry at 25°C Dry under conditions fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com