Treatment for myelosuppression
A technology of bone marrow suppression and treatment plan, applied in the direction of treatment, radiotherapy, medical preparations containing active ingredients, etc., can solve the problems of life-threatening patients, reduction of treatment dose, side effects of treatment plan, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
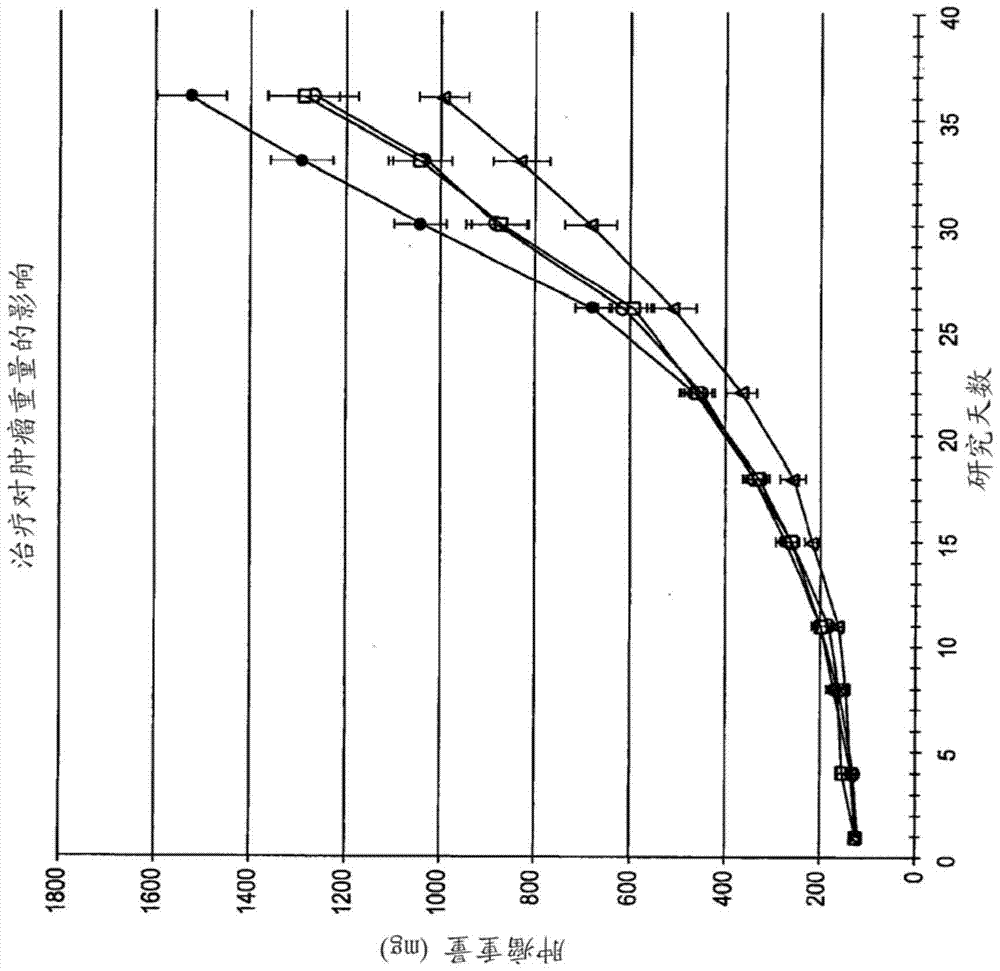
[0243] 6.2 Example 2: ODSH, a heparanoid that interacts with PF4, enhances the efficacy of carboplatin in a mouse xenograft model of human ovarian cancer
[0244] This example demonstrates that adjuvant administration of ODSH enhances the efficacy of carboplatin against human ovarian tumors grown as xenografts in athymic nude mice.
[0245] Materials & Methods ODSH (50 mg / mL stock concentration) was prepared by Pyramid Laboratories, Inc. and stored at room temperature until use. As described further below, ODSH can be administered either intravenously (IV) at a dose of 48 mg / kg and a volume of 10 mL / kg or subcutaneously (SC) at a dose of 24 mg / kg and a volume of 5 mL / kg. Carboplatin obtained from clinical suppliers was stored at 4°C until use. Carboplatin was administered by intraperitoneal injection (IP) at a dose of 80 mg / kg and a volume of 10 mL / kg. Saline solution (0.9% NaCl) was used as a vehicle control for ODSH and was administered by the same route and in the same vo...
Embodiment 3
[0261] 6.3 Example 3: ODSH, a heparanoid that interacts with PF4, alleviates thrombocytopenia and neutropenia, induces thrombopoiesis and neutrophil production and reduces exposure to chemotherapy regimens with myelosuppressive side effects Patient's systemic symptoms
[0262] In an unblinded clinical trial, patients diagnosed with metastatic pancreatic cancer were treated with ODSH as gemcitabine and nab-paclitaxel ( Adjuvant therapy with albumin-bound paclitaxel).
[0263] Inclusion criteria Male and non-pregnant, non-lactating female patients aged 18 to 75 years with histologically confirmed metastatic adenocarcinoma participated in the trial. Other inclusion criteria were: presence of at least one metastatic tumor (measurable by conventional techniques or CT scan), serum CA 19-9 measurement greater than 2 times the upper limit of normal, no local targeted therapy within six months of participation in the trial Radiotherapy or chemotherapy for advanced disease, absolute ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com