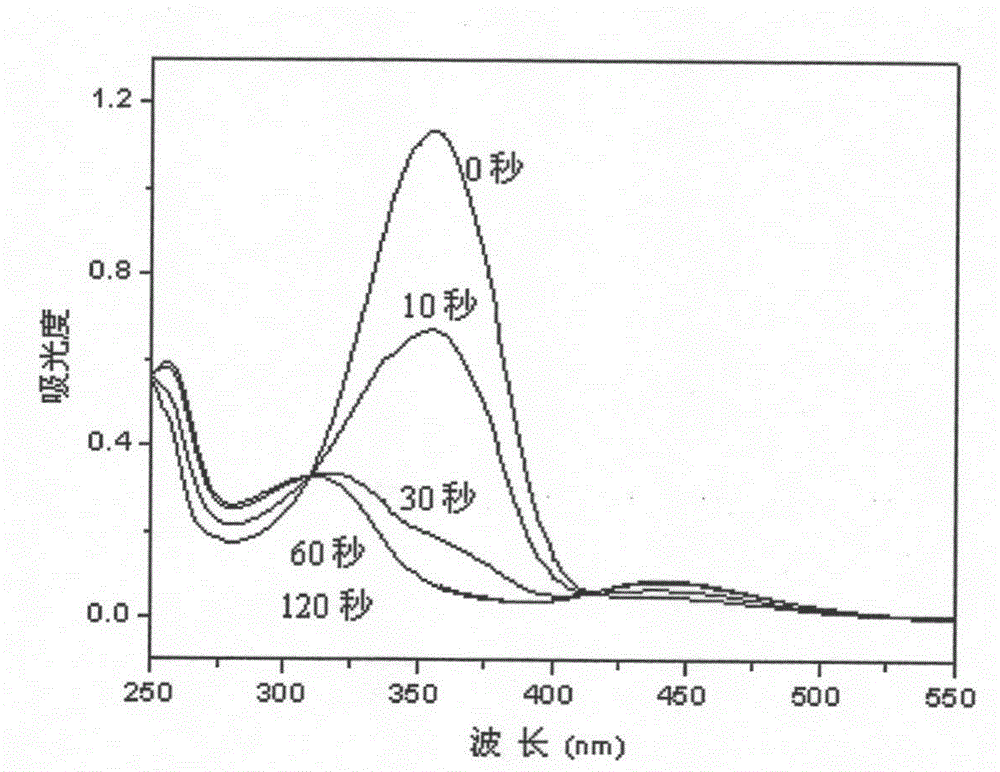
Rapid-response photoisomerization 4-perfluoroalkyl azobenzene allyl ether and preparation method thereof
A fluoroalkyl azophenyl propenyl ether, photoisomerization technology, applied in the field of azo photochromic materials, can solve the problem of slow light response speed, etc., and achieve the effect of rapid response and wide application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
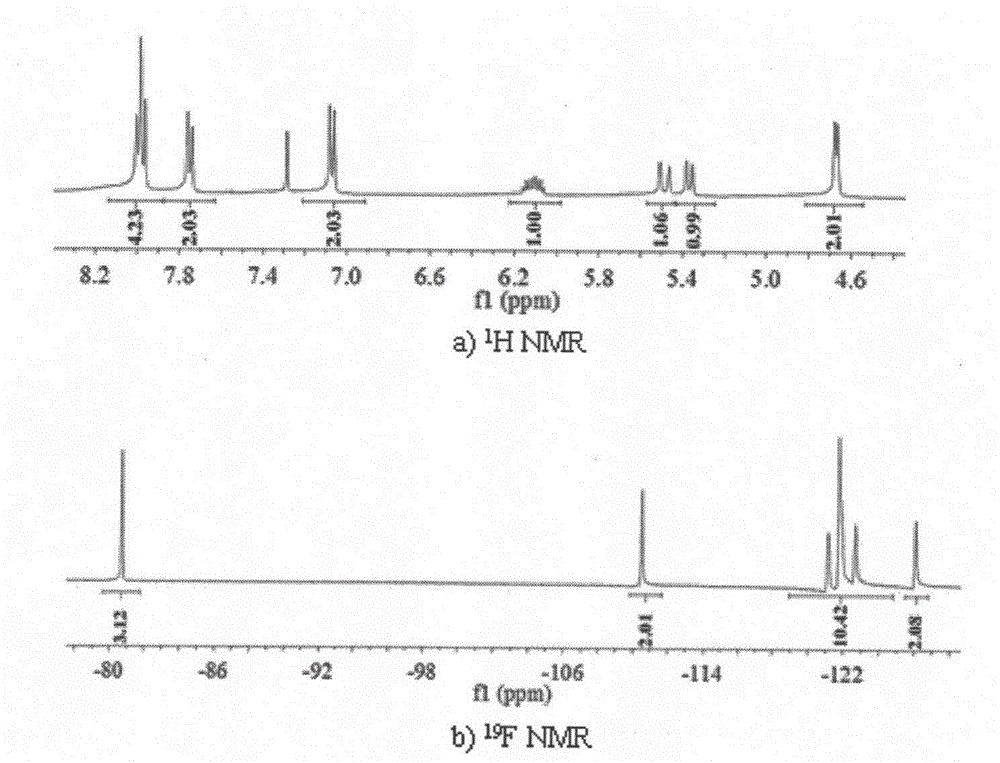
[0032] 1. Preparation of 4-perfluorooctyl azophenyl propenyl ether
[0033] (1) Add copper powder (0.9616g) to a three-necked bottle equipped with a reflux device to remove water and oxygen and fill with nitrogen for protection, and add p-iodoaniline (1.0952g) and perfluorooctyl iodide under the condition of magnetic stirring at 120°C Alkanes (3.0032g) in DMSO solution 15mL, react for 5-10 hours until the reaction is complete. After the reaction, filter to remove excess copper powder and cuprous iodide, wash the reaction solution with 50mL ether and 50mL aqueous solution, wash the ether layer three times with 50mL aqueous solution to remove DMSO and excess perfluorooctyl iodide, and decompress the washed ether layer It was spin-dried and purified by column chromatography to obtain perfluorooctylaniline (colorless solid, yield 81.5%).
[0034] (2) Add 1.5mL of 12M concentrated hydrochloric acid dropwise to 1.0224g of perfluorooctylaniline under ice bath conditions (0-5°C), dis...
Embodiment 2
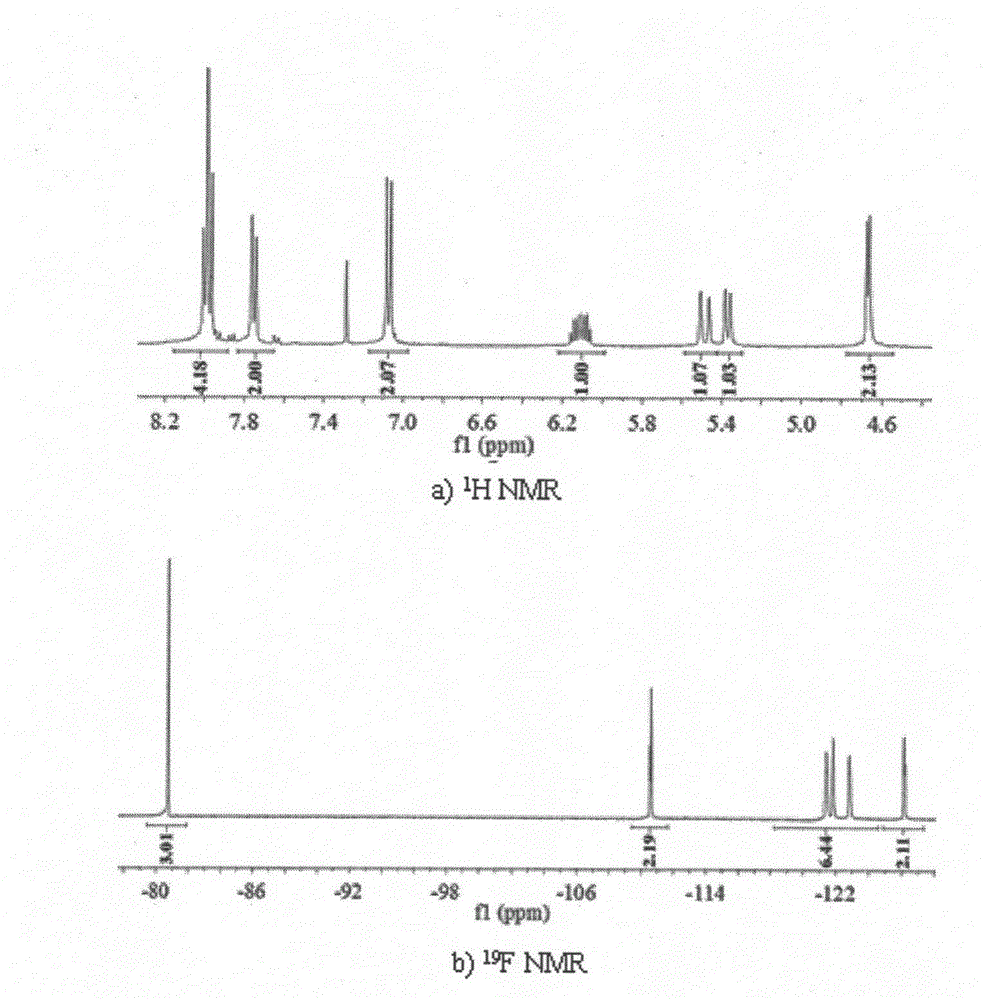
[0039] 1. Preparation of 4-perfluorohexyl azophenyl propenyl ether
[0040] (1) Add copper powder (0.9704g) to a three-necked bottle equipped with a reflux device to remove water and oxygen and fill with nitrogen for protection, and add p-iodoaniline (1.0875g) under magnetic stirring conditions at 120°C, and perfluorohexyl iodide (2.4538g) in 15 mL of DMSO solution, react for 5-10 hours until the reaction is complete. After the reaction, filter to remove excess copper powder and cuprous iodide, wash the reaction solution with 50mL ether and 50mL aqueous solution, wash the ether layer three times with 50mL aqueous solution to remove DMSO and excess perfluorooctyl iodide, and decompress the washed ether layer It was spin-dried and purified by column chromatography to obtain perfluorohexylaniline (colorless solid, yield 84.27%).
[0041] (2) Add 1.5mL of 12M concentrated hydrochloric acid dropwise to perfluorohexylaniline (0.8226g) under ice bath conditions (0-5°C), dissolve und...
Embodiment 3
[0047] 1. Preparation of 4-perfluorobutyl azophenyl propenyl ether
[0048] (1) Add copper powder (0.9654g) to a three-necked bottle equipped with a reflux device to remove water and oxygen and fill with nitrogen for protection, and add p-iodoaniline (1.0936g) under magnetic stirring conditions at 120°C, and perfluorobutyl iodide Alkanes (1.9246g) in DMSO solution 15mL, react for 5-10 hours until the reaction is complete. After the reaction, filter to remove excess copper powder and cuprous iodide, wash the reaction solution with 50mL ether and 50mL aqueous solution, wash the ether layer three times with 50mL aqueous solution to remove DMSO and excess perfluorooctyl iodide, and decompress the washed ether layer Spin to dry and purify by column chromatography to obtain perfluorobutylaniline (colorless liquid, yield 86.52%).
[0049] (2) Add 1.5mL of 12M concentrated hydrochloric acid dropwise to perfluorobutylaniline (0.6248g) under ice bath conditions (0-5°C), dissolve under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com