Reaction type halamine antiseptic, preparation method, and applications thereof
An antibacterial agent and a reactive technology, which are applied in the field of reactive halamine antibacterial agents and their preparation, can solve the problems of poor water solubility of the antibacterial agent precursor, and poor washing and regeneration performance, and achieve excellent bacteriostatic and sterilizing functions. , low price, the effect of improving antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
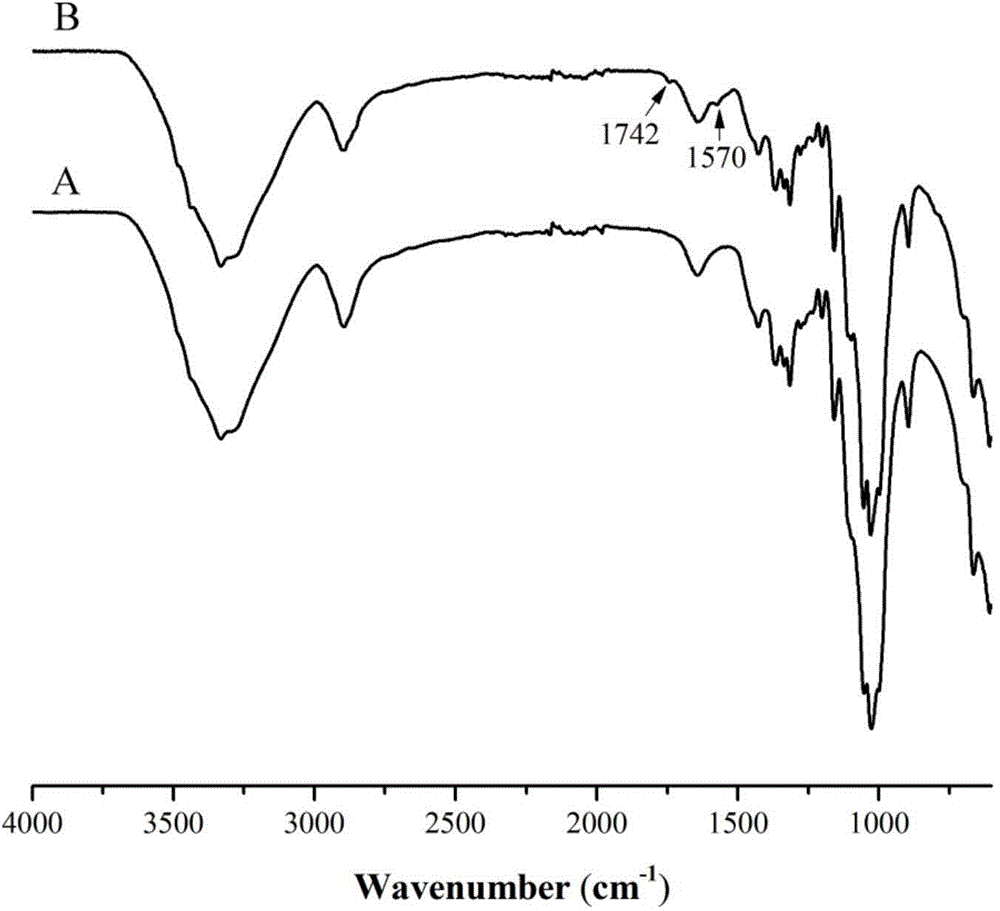
Image
Examples
Embodiment 1
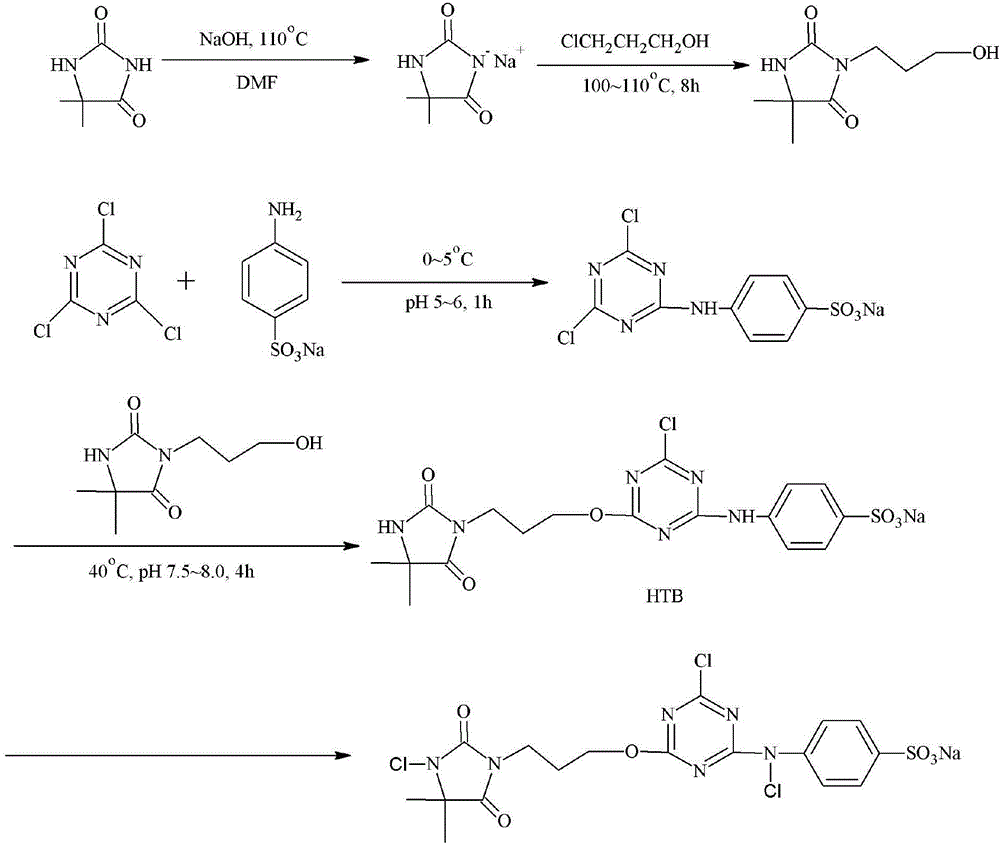
[0039] Haloamine antibacterial agent precursor 2-chloro-4-(4-sulfoanilino)-6-(3-hydroxypropyl-5,5-dimethylhydantoin)-1,3,5-tri Preparation of oxazine:
[0040] Weigh 6.4g of 5,5-dimethylhydantoin and 2.8g of potassium hydroxide and reflux in water for 10min, then add 4.73g of 3-chloro-1-propanol, continue the reaction for 12h, and remove the moisture by distillation under reduced pressure after the end. After washing with acetone, remove potassium chloride by filtration, distill under reduced pressure, and dry.
[0041] Weigh 9.22g cyanuric chloride, dissolve it in 100mL carbon tetrachloride, weigh 8.66g p-aminobenzenesulfonic acid and 2.65g sodium carbonate, dissolve it in 60mL water, mix the two in a 250mL three-necked flask and placed in an ice bath, and reacted for 1 hour at a pH value of 5.0 to 6.0, then poured 50 mL of the 3-hydroxypropyl-5,5-dimethylhydantoin solution obtained above into the reaction liquid, and the pH The value is 7.0-8.0, and the temperature is 40° ...
Embodiment 2
[0043] Haloamine antibacterial agent precursor 2-chloro-4-(4-sulfoanilino)-6-(3-hydroxypropyl-5,5-dimethylhydantoin)-1,3,5-tri Preparation of oxazine:
[0044] Weigh 6.4g of 5,5-dimethylhydantoin and 2g of sodium hydroxide and reflux in ethanol for 10min, then add 4.73g of 3-chloro-1-propanol and continue the reaction for 24h. After washing with ethanol, filter sodium chloride, distill under reduced pressure, and dry.
[0045] Weigh 9.22g of cyanuric chloride, dissolve it in 100mL of chloroform, then pour 50mL of the 3-hydroxypropyl-5,5-dimethylhydantoin solution obtained above into it, at a pH of 7.0 to 8.0 React under the conditions for 3 hours, then weigh 8.66g of p-aminobenzenesulfonic acid and 2.65g of sodium carbonate and dissolve them in 50mL of water, pour them into the above reaction solution, and react for 6 hours at a pH value of 5.0-6.0 and a temperature of 30°C. After the end, filter, wash with acetone and ice water, then dry and store.
Embodiment 3
[0047] Haloamine antibacterial agent precursor 2-fluoro-4-(4-sulfoanilino)-6-(3-hydroxypropyl-5,5-dimethylhydantoin)-1,3,5-tri Preparation of oxazine:
[0048] Weigh 6.4g of 5,5-dimethylhydantoin and 2g of sodium hydroxide in N,N-dimethylformamide, stir at 95°C for 10min, then add 4.73g of 3-chloro-1-propanol , continue the reaction for 18h, after the completion of vacuum distillation to remove N,N-dimethylformamide, wash with ethanol, filter sodium chloride, vacuum distillation, and dry.
[0049] Weigh 9.22g of cyanuric fluoride, dissolve it in 100mL of acetone, weigh 8.66g of p-aminobenzenesulfonic acid and 2.65g of sodium carbonate, dissolve it in 60mL of water, mix the two in a 250mL three-necked flask and place them together In an ice bath, react for 2 hours at a pH value of 5.0 to 6.0, then pour 50 mL of the 3-hydroxypropyl-5,5-dimethylhydantoin aqueous solution obtained above into it, and pour it into the above reaction The solution was reacted at a pH value of 7.0-8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com