A kind of Fritillaria superfine powder and its preparation method and application
An ultra-fine powder, fritillary technology, applied in antibacterial drugs, powder delivery, pharmaceutical formulations, etc., to improve the immune function of patients, the stability of the patient's condition, and improve the quality of life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Fritillaria superfine powder
[0034] Step 1: Select and clean the materials, select the pearl oysters in the fritillaria of the right size, remove impurities, and the impurity content should not exceed 1%, put the fritillaria after washing and cleaning in the cleaning tank until there is no sediment on the surface until.
[0035] Step 2: dry and sterilize, put the cleaned Fritillaria in a drying room for drying, the temperature of the drying treatment is 85°C, and the drying time is 6 hours; the dried Fritillaria is sterilized, and the sterilized The temperature is 120°C, and the sterilization time is 0.5 hours. Because the pearl oyster in the Fritillaria fritillaria selected for use does not need to remove the core bud when making medicine, under the above-mentioned drying conditions, the moisture of the pearl oyster will volatilize more, and the drying effect is the best. The sterilization condition is set at 120°C and the time is 0.5 hours. At this ...
Embodiment 2
[0041] Preparation of Fritillary Granules
[0042] The Fritillaria superfine powder obtained in Example 1 was added with auxiliary material β-cyclodextrin to make 5000 g of Fritillaria granules, wherein the mass ratio of Fritillaria superfine powder to β-cyclodextrin was 2:1. Among the excipients, Fritillaria and β-cyclodextrin have the best combination effect, and the quality ratio has better drug effect, better taste, and is easier for patients to accept. Among the granules, Fritillaria superfine powder is the only active ingredient.
Embodiment 3
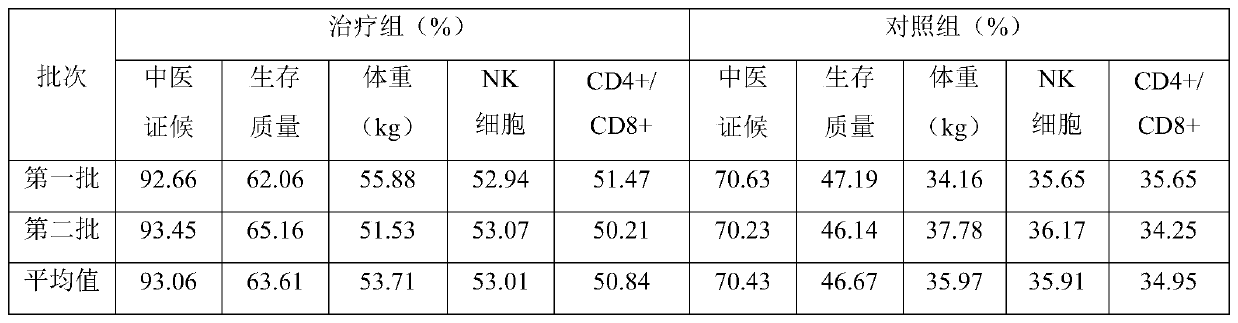
[0044] Clinical Trials
[0045] 1 Materials and methods
[0046] 1.1 Test drugs
[0047] 1.1.1 The prepared Fritillaria granules of embodiment 2
[0048] 1.1.2 Drugs used in the control group
[0049] Drugs purchased from
[0050] Cisplatin Jiangsu Hansoh Pharmaceutical Co., Ltd.
[0051] Pingyangmycin Zhejiang Hisun Pharmaceutical Co., Ltd.
[0052] Bleomycin Zhejiang Hisun Pharmaceutical Co., Ltd.
[0053] Epirubicin Pfizer Pharmaceuticals (Wuxi) Co., Ltd.
[0054] Vincristine Sulfate Yangzhou Aosaikang Pharmaceutical Co., Ltd.
[0055] Vindesine Sulfate Hangzhou Minsheng Pharmaceutical Group Co., Ltd.
[0056] Paclitaxel Yangzijiang Pharmaceutical Group Co., Ltd.
[0057] Cyclophosphamide Shanghai Huashi Pharmaceutical Co., Ltd. Tianping Pharmaceutical Factory
[0058] Ifosfamide Jiangsu Hengrui Medicine Co., Ltd.
[0059] Prednisone Chifeng Mengxin Pharmaceutical Co., Ltd.
[0060] Interferon-a Beijing Kain Biotechnology Co., Ltd.
[0061] Interleukin-2 Shenzh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com