Method for increasing the concentration of sodium sulfide solution by leaching black ash three times
A technology of solution concentration and sodium sulfate solution, applied in chemical instruments and methods, alkali metal sulfide/polysulfide, inorganic chemistry, etc., can solve the problems of low concentration of sodium sulfide solution and high cost of 60 alkali evaporation, and reduce evaporation The effect of increasing the concentration of sodium sulfide solution and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The resulting barium sulfide and sodium sulfate solution reacted to form barium sulfate and sodium sulfide solution after soaking black ash with water, the concentration of sodium sulfide solution is 4%.
[0017] The black ash was leached three times with a sodium sulfide solution with a concentration of 4%, and the steps were as follows:
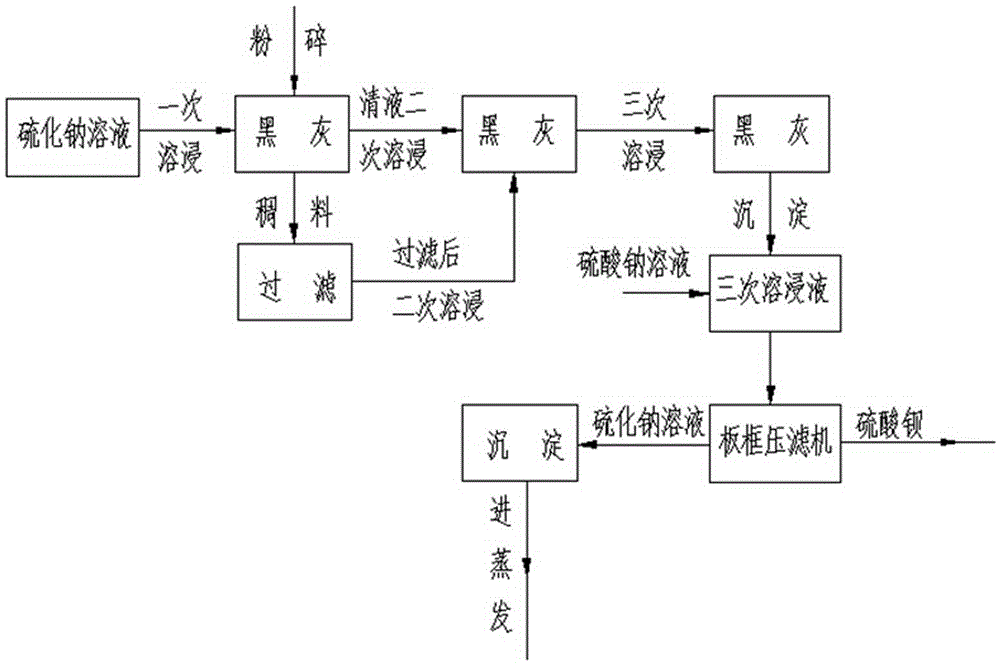
[0018] ① first crush the black ash, and then use 4% sodium sulfide solution for a leaching; ② precipitate and separate the leached solution to obtain the mother liquor I; ③ use the mother liquor Ⅰ to continue the second leaching of the black ash, The leached solution is subjected to secondary precipitation and separation to obtain mother liquor II; ④ three times of leaching with mother liquor II, and the solution after three leachings is subjected to precipitation and separation to obtain mother liquor III; ⑤ mother liquor III contains S 2- 、Na + 、Ba 2+ ions, react with sodium sulfate solution to form barium sulfate and sodium sulf...
Embodiment 2
[0024] The resulting barium sulfide and sodium sulfate solution reacted to form barium sulfate and sodium sulfide solution after soaking black ash with water, the concentration of sodium sulfide solution is 5%.
[0025] The black ash is leached three times with sodium sulfide solution, the steps are as follows:
[0026] ① first crush the black ash, and then use 5% sodium sulfide solution for a leaching; ② precipitate and separate the leached solution to obtain the mother liquor I; ③ use the mother liquor Ⅰ to continue the second leaching of the black ash, The leached solution is subjected to secondary precipitation and separation to obtain mother liquor II; ④ three times of leaching with mother liquor II, and the solution after three leachings is subjected to precipitation and separation to obtain mother liquor III; ⑤ mother liquor III contains S 2- 、Na + 、Ba 2+ ions, react with sodium sulfate solution to form barium sulfate and sodium sulfide solution; ⑥The concentration of...
Embodiment 3
[0032] The resulting barium sulfide and sodium sulfate solution reacted to form barium sulfate and sodium sulfide solution after soaking black ash with water, the concentration of sodium sulfide solution is 4.8%.
[0033] The black ash is leached three times with sodium sulfide solution, the steps are as follows:
[0034] ① first crush the black ash, and then use 4.8% sodium sulfide solution for a leaching; ② precipitate and separate the leached solution to obtain the mother liquor Ⅰ; ③ use the mother liquor Ⅰ to continue to leaching the black ash twice, The leached solution is subjected to secondary precipitation and separation to obtain mother liquor II; ④ three times of leaching with mother liquor II, and the solution after three leachings is subjected to precipitation and separation to obtain mother liquor III; ⑤ mother liquor III contains S 2- 、Na + 、Ba 2+ ions, react with sodium sulfate solution to form barium sulfate and sodium sulfide solution; ⑥The concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com