Industrial preparation method of micronized iloperidone
A micronization technology of iloperidone, which is applied in the field of preparation of iloperidone ultrafine powder, and achieves the effects of safe operation, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
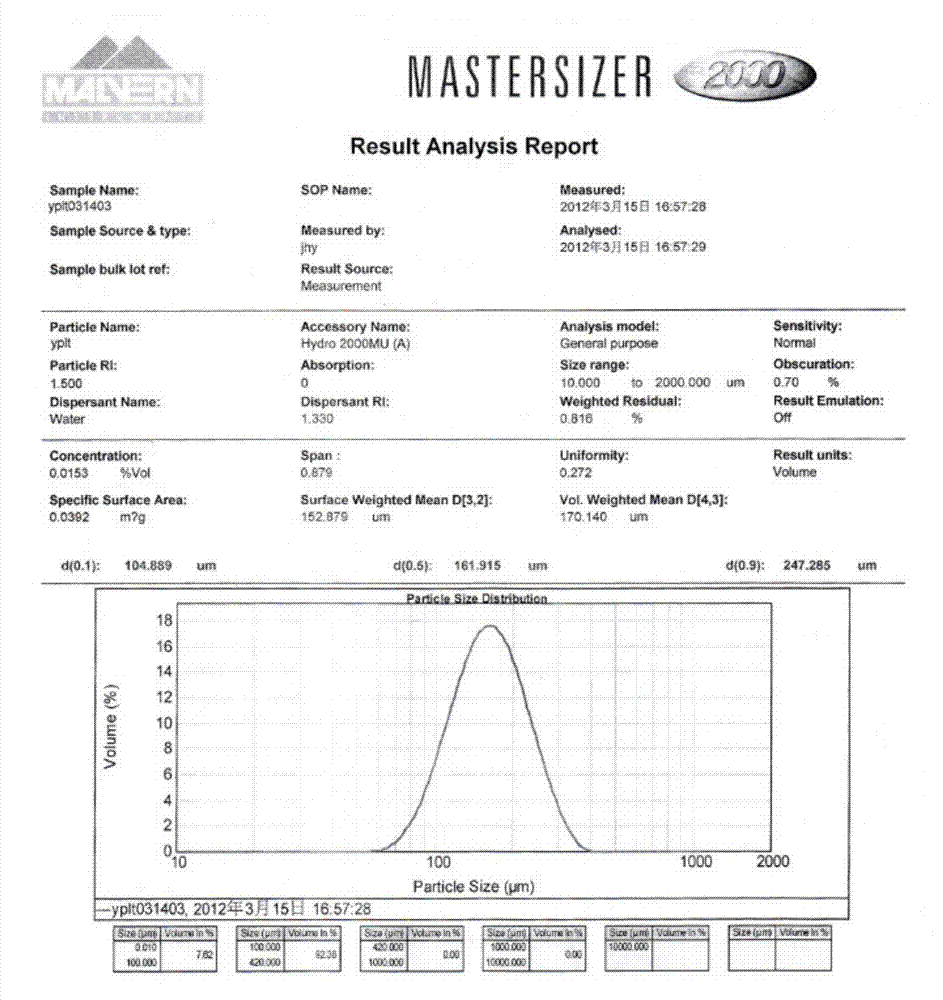
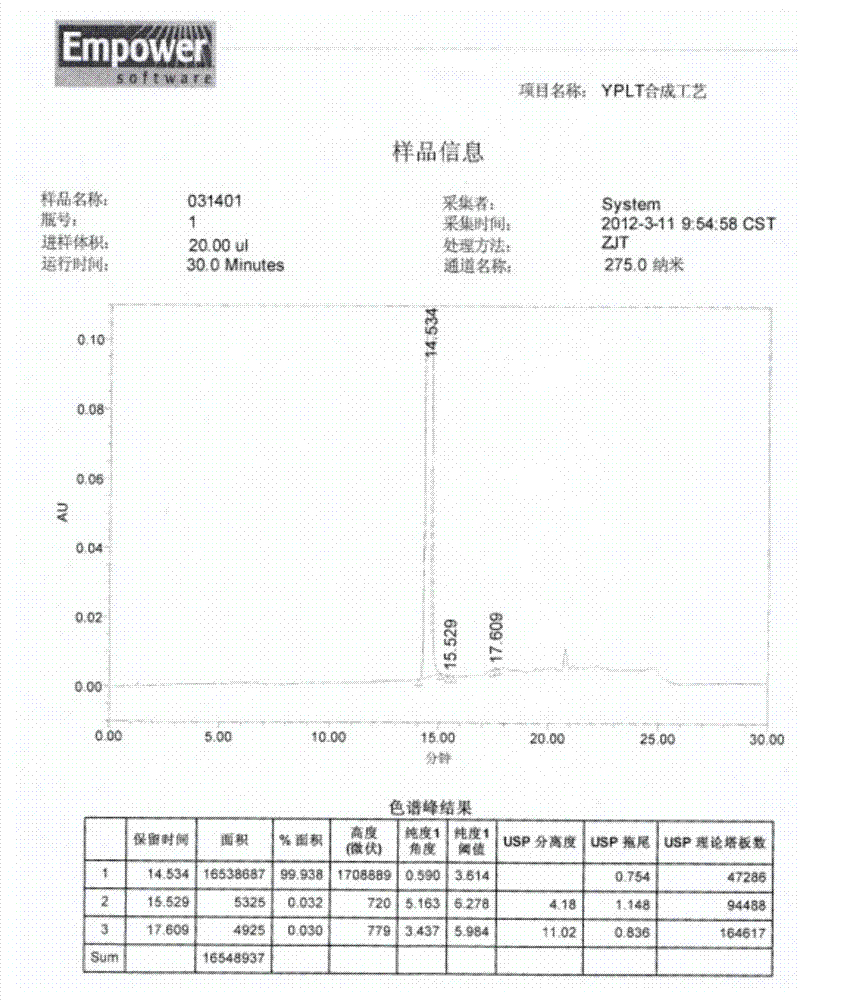
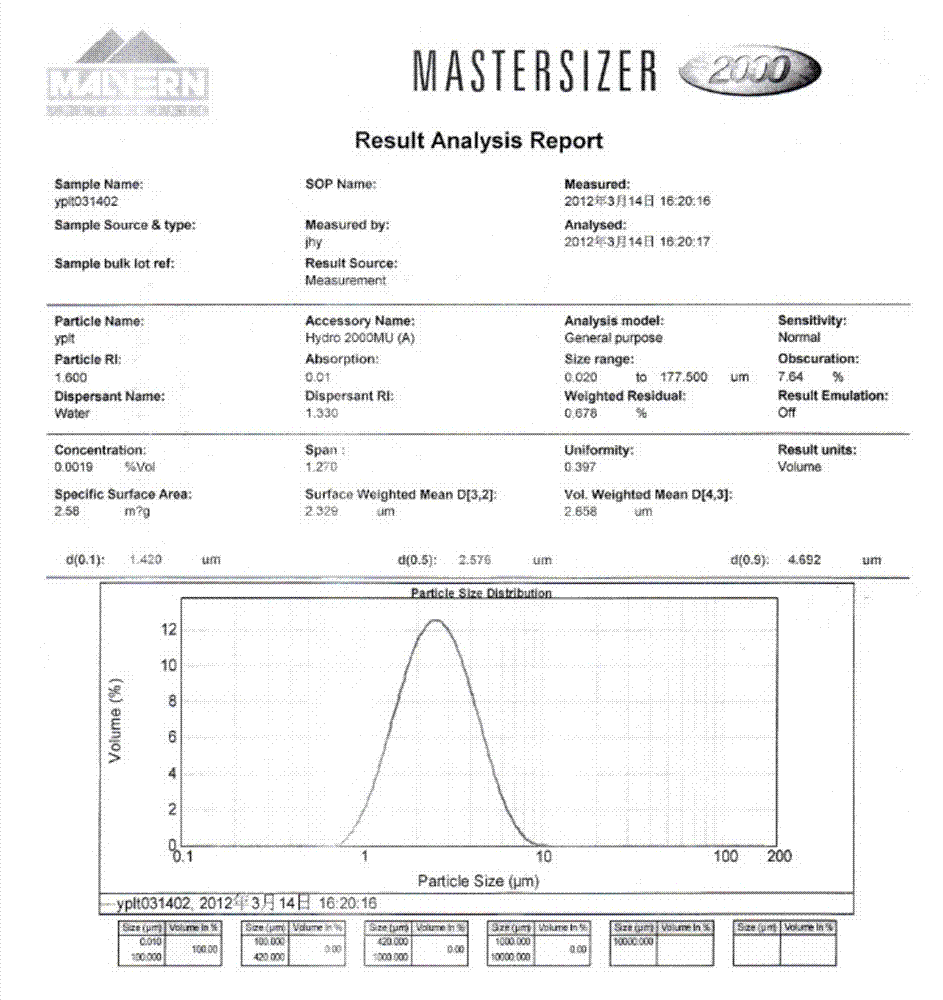
[0051] Add 100g of commercially available iloperidone raw material drug into 500ml of methanol, heat to reflux to dissolve, add the methanol solution of iloperidone into 5000ml of water at 50°C at a stirring speed of 1000rpm, cool to 30°C and age for 2h, filter , dried at 50-60°C to obtain the micronized iloperidone product, the particle size test is as follows: image 3 Shown, the product average particle size D 90 =4.692μm, purity 99.975%, the chromatogram of related substances is as follows Figure 4 .
Embodiment 2
[0053]Add 100g of commercially available iloperidone raw material drug into 1000ml of ethanol, heat to reflux to dissolve, add the ethanol solution of iloperidone into 10000ml of 50°C water at a stirring speed of 1000rpm, cool down to 40°C and age for 3h, then filter , dried at 50-60°C to obtain the micronized iloperidone product, tested by particle size analyzer as Figure 5 Shown, the product average particle size D 90 =4.996μm, purity 99.974%, the chromatogram of related substances is as follows Figure 6 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com