Noninvasive human liver cancer early detection and differential diagnosis method and system
A technology for early detection and differential diagnosis, applied in the field of clinical medicine, can solve the problems of imaging examinations that cannot be used for early diagnosis, serological tests lack specificity, and cannot be used for early diagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Genome CNV analysis of cell-free peripheral blood DNA
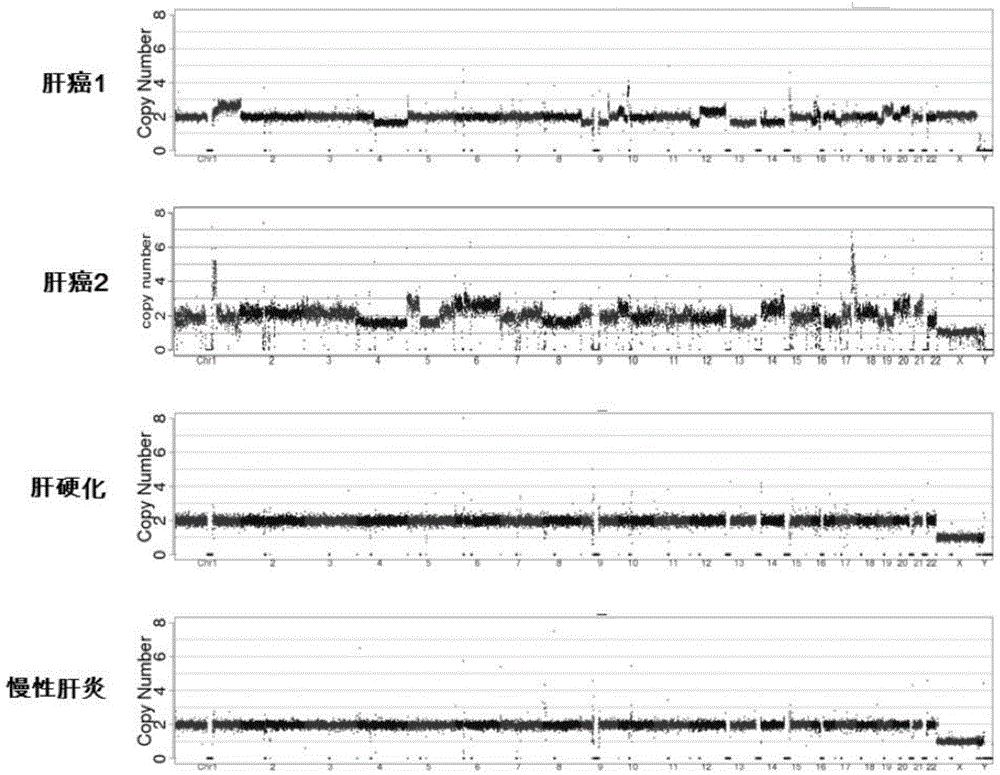
[0024] The isolated plasma of 31 cases of clinically diagnosed liver cancer patients and 8 cases of isolated plasma of patients with chronic hepatitis and cirrhosis were collected, and the free DNA in the plasma was extracted, and the genome sequencing library was constructed with the kit of NEB Company. The DNA of each library was initially The dosage is 3-5ng. The Illumina Hiseq2500SR41 method was used for genomic DNA sequencing, and the effective sequencing number of each sample was 10M. BWA software was used for sequence comparison, and then CNV analysis was performed according to the steps of the present invention, wherein the CNV normal control data used came from 250 normal people.
[0025] As a result of the measurement, it was found that some liver cancer patients (41.9%, 13 / 31) had observed abnormal changes in the chromosome CNV map, and the chronic hepatitis and liver cirrhosis group had no v...
Embodiment 2
[0026] Embodiment 2: the establishment of Z-score CNV comprehensive scoring method
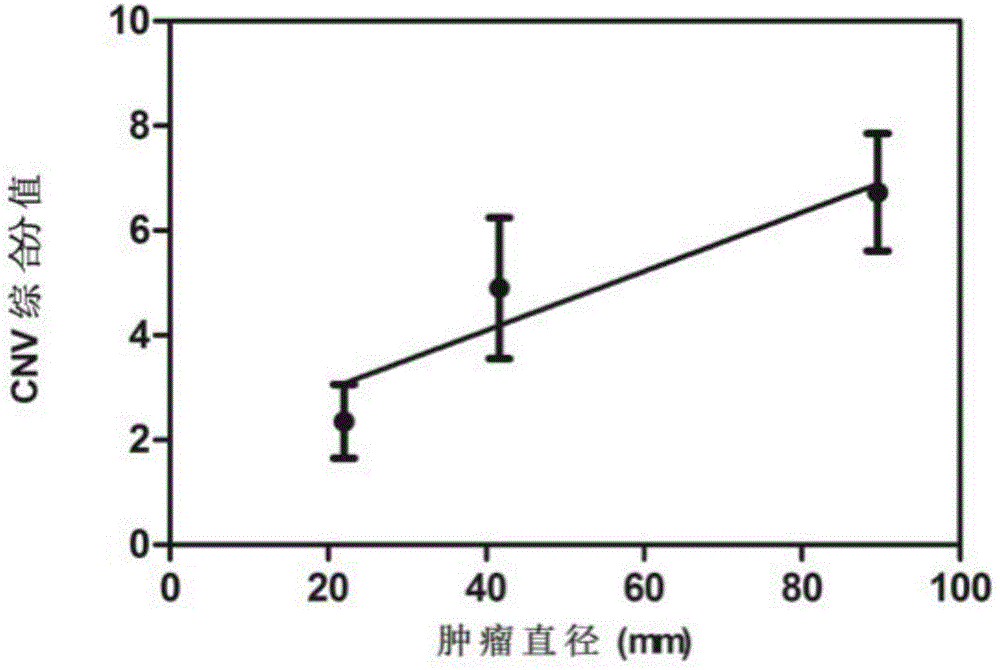
[0027] Score according to the following principles: 4 points for visually observable copy number changes on the chromosome CNV map, 2 points for 1q, 7q, or 19q copy number increase in the above-mentioned Z-score CNV changes, and 2 points for the above-mentioned 1p, 9q, or 14q CNV changes The copy number reduction is 0.5 points, and 1.5 is the critical value, higher than or equal to 1.5 is high risk of liver cancer, and less than 1.5 is low risk of liver cancer.
[0028] The high risk of liver cancer indicates that the possibility of liver cancer is high, especially on the basis of chronic hepatitis and cirrhosis. It is necessary to follow closely and use other methods to verify the existence of liver cancer, so as to achieve the purpose of early diagnosis and early treatment. The CNV composite score is correlated with the size of liver cancer, see figure 2 .
Embodiment 3
[0029] Example 3: Application of CNV Comprehensive Score in Early Detection and Differential Diagnosis of Liver Cancer
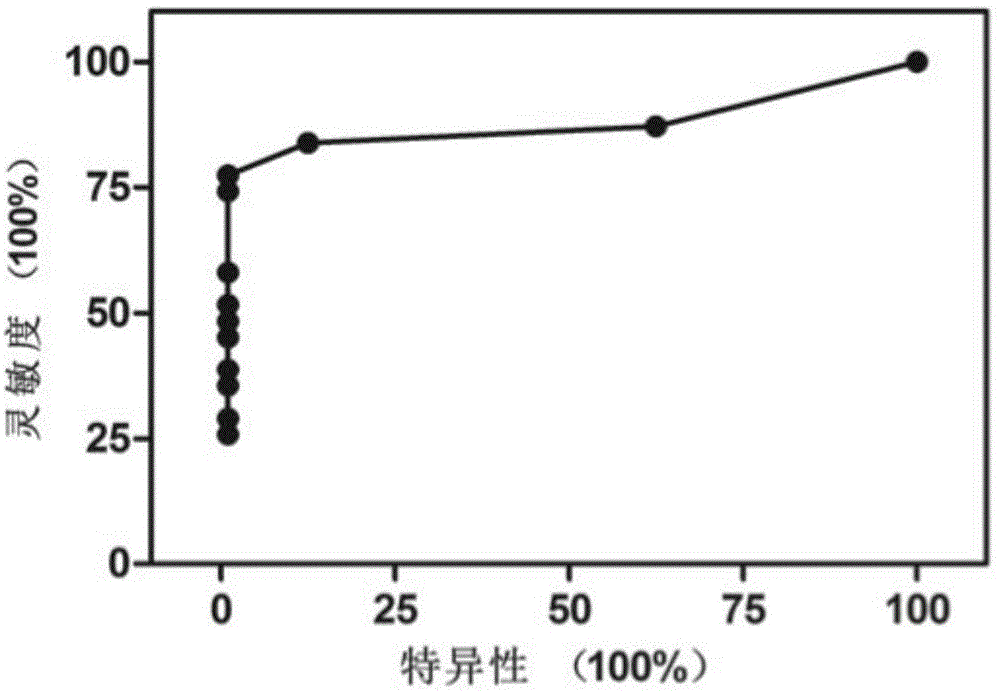
[0030] The above-mentioned CNV comprehensive scoring method analyzes liver cancer and chronic hepatitis liver cirrhosis measurement data, and the positive rate of the liver cancer group is 83.9% (26 / 31), the positive rate of the liver cancer group below 50mm is 68.8% (11 / 16), and the positive rate of the liver cancer group below 30mm The positive rate was 57.1% (4 / 7), the positive rate was 70% (7 / 10) in the liver cancer group with negative serum AFP or lower level (less than 50ng / ml), and all negative in the hepatitis cirrhosis group (0 / 8 ), the overall sensitivity was 84%, and the specificity was 100%. ROC plot see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com