Copolymer donor material for optically active layer of polymer solar cell (PSC) and preparation method of copolymer donor material
A technology of solar cells and photoactive layers, which is applied in the fields of electrical solid-state devices, semiconductor/solid-state device manufacturing, circuits, etc., can solve problems such as low conjugation, low band gap, and poor solubility, and achieve enhanced solubility and processing Performance, increased conjugation degree, effect of high conjugation degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Synthesis of 2-hydroxy-2-(4-methylphenyl)acetic acid
[0031] Add 4-methylbenzaldehyde (0.1 mol), chloroform (0.2 mol) and benzyltriethylammonium chloride (0.2 mol) with a molar ratio of 1: 2: 2 in a 250 mL three-necked flask, and then slowly 5 mL of 50% sodium hydroxide aqueous solution was added dropwise, and the temperature was controlled at 52 ℃ to react for 7 h. After the reaction, the reaction mixture was poured into 50 mL deionized water to dilute, and extracted twice with ether. The aqueous layer was acidified with sulfuric acid to pH 1, and then extracted twice with ether. After the ether was evaporated from the extract, it was vacuum dried to obtain 2-hydroxy-2-(4-methylphenyl)acetic acid with a yield of 91%.
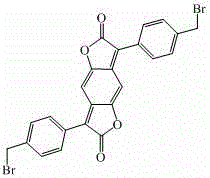
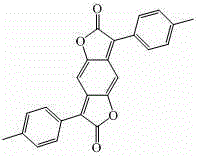
[0032] 2) Synthesis of 3,7-bis-(4-methylphenyl)benzo[1,2-b:4,5-b’]difuran-2,6-dione
[0033] Add hydroquinone (20 mmol), p-methylmandelic acid (100 mmol), and sulfuric acid (40 mmol) in a molar ratio of 1:5:2 to a 250 mL three-necked flask. Add 20 mL of ...
Embodiment 2
[0048] 1) Synthesis of 2-hydroxy-2-(4-methylphenyl)acetic acid
[0049] Add 4-methylbenzaldehyde (0.1 mol), chloroform (0.2 mol) and benzyltriethylammonium chloride (0.3 mol) with a molar ratio of 1: 2: 3 in a 250 mL three-necked flask, and then slowly 7 mL of 50% sodium hydroxide aqueous solution was added dropwise, and the temperature was controlled at 55 ℃ to react for 9 h. After the reaction, the reaction mixture was diluted with 80 mL of deionized water, and extracted twice with ether. The aqueous layer was acidified with sulfuric acid to pH 1, and then extracted twice with ether. After the ether was evaporated from the extract, it was vacuum dried to obtain 2-hydroxy-2-(4-methylphenyl)acetic acid with a yield of 94%.
[0050] 2) Synthesis of 3,7-bis-(4-methylphenyl)benzo[1,2-b:4,5-b’]difuran-2,6-dione
[0051] Add hydroquinone (20 mmol), p-methylmandelic acid (100 mmol), and sulfuric acid (80 mmol) in a molar ratio of 1:5:4 to a 250 mL three-necked flask. Add 25 mL of acetic...
Embodiment 3
[0066] 1) Synthesis of 2-hydroxy-2-(4-methylphenyl)acetic acid
[0067] Add 4-methylbenzaldehyde (0.1 mol), chloroform (0.3 mol) and benzyltriethylammonium chloride (0.4 mol) with a molar ratio of 1:3:4 to a 250 mL three-necked flask, and then slowly 10 mL of 50% sodium hydroxide aqueous solution was added dropwise, and the temperature was controlled at 60 ℃ to react for 10 h. After the reaction, the reaction mixture was diluted with 90 mL of deionized water, and extracted twice with ether. The aqueous layer was acidified with sulfuric acid to pH 2, and then extracted twice with ether. After the ether was evaporated from the extract, it was vacuum dried to obtain 2-hydroxy-2-(4-methylphenyl)acetic acid with a yield of 90%.
[0068] 2) Synthesis of 3,7-bis-(4-methylphenyl)benzo[1,2-b:4,5-b’]difuran-2,6-dione
[0069] Add hydroquinone (20 mmol), p-methylmandelic acid (200 mmol), and sulfuric acid (80 mmol) in a molar ratio of 1:10:4 to a 250 mL three-necked flask. Add 30 mL of aceti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bandgap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com