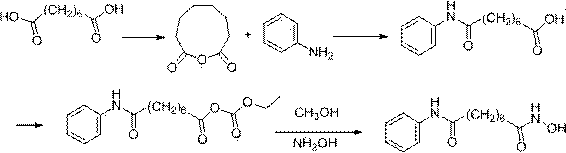
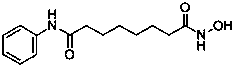
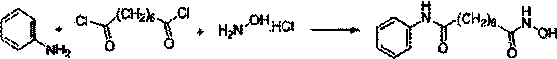Method for synthesizing anti-cancer drug vorinostat
A technology for vorinostat and anticancer drugs, applied in the field of drug synthesis, can solve problems such as long time consumption, difficulty in vorinostat, and non-production operation, and achieve the effects of good yield, high purity and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Suberic Acid to Octanoanilide
[0041] Add suberic acid (1.74g, 10mmol) into a 20ml tetrahydrofuran reaction flask, then add 6.7g perfluorosulfonic acid resin, stir for 10-30 minutes, add aniline (0.93g, 10mmol), stir, and heat to 75°C, there is a slight acetonitrile reflux at this time, keep this state for 5 hours, TLC detects the reaction, after the reaction is completed, filter and remove the perfluorosulfonic acid resin, and wash the perfluorosulfonic acid resin with a small amount of acetonitrile 1-2 times, recover spare. Concentrate the filtrate to obtain a concentrate, then add 15ml of 1mol / L aqueous sodium hydroxide solution to the concentrate, stir at 40-50°C for 10-30 minutes, then filter while hot to remove all insolubles, heat the obtained filtrate, and Add HCl solution dropwise to the obtained filtrate, adjust the pH=1-2, then cool to 20°C-25°C, crystallize, filter to obtain a solid, and then wash twice with 20-25°C pure water, 20ml each time , and then d...
Embodiment 2
[0045] Suberic Acid to Octanoanilide
[0046]Add suberic acid (1.74g, 10mmol) into a 20ml acetonitrile reaction flask, then add 5.3g perfluorosulfonic acid resin, stir for 10-30 minutes, add aniline (0.93g, 10mmol), stir, and heat to 80°C, there is slight acetonitrile reflux at this time, keep this state for 5 hours, TLC detects the reaction, after the reaction is completed, filter and remove the perfluorosulfonic acid resin, and wash the perfluorosulfonic acid resin with a small amount of acetonitrile 1-2 times, recover spare. Concentrate the filtrate to obtain a concentrate, then add 15ml of 1mol / L aqueous sodium hydroxide solution to the concentrate, stir at 40-50°C for 10-30 minutes, then filter while hot to remove all insolubles, heat the obtained filtrate, and Add HCl solution dropwise to the obtained filtrate, adjust the pH=1-2, then cool to 20°C-25°C, crystallize, filter to obtain a solid, and then wash twice with 20-25°C pure water, 20ml each time , and then dried i...
Embodiment 3
[0050] Suberic Acid to Octanoanilide
[0051] Add suberic acid (17.4g, 0.1mol) into a 200ml acetonitrile reaction flask, then add 53g perfluorosulfonic acid resin, stir for 10-30 minutes, add aniline (9.3g, 0.1mol), stir, and heat to react To 80°C, there is a slight acetonitrile reflux at this time, keep this state for 5 hours, TLC to detect the reaction, after the reaction is completed, filter out the perfluorosulfonic acid resin, and wash the perfluorosulfonic acid resin with a small amount of acetonitrile 1-2 times, Recycle for spare. Concentrate the filtrate to obtain a concentrate, then add 150ml of 1mol / L aqueous sodium hydroxide solution to the concentrate, stir at 40-50°C for 10-30 minutes, then filter while hot to remove all insolubles, heat the obtained filtrate, and Add HCl solution dropwise to the obtained filtrate, adjust the pH=1-2, then cool to 20°C-25°C, crystallize, filter to obtain a solid, and then wash twice with 20-25°C pure water, 200ml each time , and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com